Sigma-2 receptor antagonists rescue neuronal dysfunction induced by Parkinson’s patient brain-derived α-synuclein
By Colleen S. Limegrover, Raymond Yurko, Nicholas J. Izzo, Kelsie M. LaBarbera, Courtney Rehak, Gary Look, Gilbert Rishton, Hank Safferstein, and Susan M. Catalano
Excerpt from the article published in Journals of Neuroscience Research; 99: 1161– 1176, 22 January 2021, DOI: https://doi.org/10.1002/jnr.24782
Editor’s Highlights
- Oligomeric α-synuclein proteins found in Parkinson’s disease patient brain tissue cause neuron dysfunction.
- α-synuclein oligomers upregulated the expression of lysosomal-associated membrane protein-2A (LAMP-2A), a protein critically required for chaperone-mediated autophagy.
- Selective sigma-2 receptor allosteric antagonists block recombinant α-synuclein oligomer-induced LAMP-2A upregulation.
- Inhibitors that modulate sigma-2 receptors may be therapeutic against oligomeric α-synuclein-induced neuronal dysfunction in PD and other α-synucleinopathies.
Abstract
α-Synuclein oligomers are thought to have a pivotal role in sporadic and familial Parkinson’s disease (PD) and related α-synucleinopathies, causing dysregulation of protein trafficking, autophagy/lysosomal function, and protein clearance, as well as synaptic function impairment underlying motor and cognitive symptoms of PD. Moreover, trans-synaptic spread of α-synuclein oligomers is hypothesized to mediate disease progression. Therapeutic approaches that effectively block α-synuclein oligomer-induced pathogenesis are urgently needed. Here, we show for the first time that α-synuclein species isolated from human PD patient brain and recombinant α-synuclein oligomers caused similar deficits in lipid vesicle trafficking rates in cultured rat neurons and glia, while α-synuclein species isolated from non-PD human control brain samples did not. Recombinant α-synuclein oligomers also increased neuronal expression of lysosomal-associated membrane protein-2A (LAMP-2A), the lysosomal receptor that has a critical role in chaperone-mediated autophagy. Unbiased screening of several small molecule libraries (including the NIH Clinical Collection) identified sigma-2 receptor antagonists as the most effective at blocking α-synuclein oligomer-induced trafficking deficits and LAMP-2A upregulation in a dose-dependent manner. These results indicate that antagonists of the sigma-2 receptor complex may alleviate α-synuclein oligomer-induced neurotoxicity and are a novel therapeutic approach for disease modification in PD and related α-synucleinopathies.
Significance
Oligomeric α-synuclein proteins found in Parkinson’s disease patient brain tissue cause neuron dysfunction, and therapeutic approaches effectively targeting them are urgently needed. For the first time, this study demonstrates that recombinant and Parkinson’s patient-derived α-synuclein cause similar lipid vesicle trafficking deficits in neurons, while α-synuclein species isolated from non-Parkinson’s human control brain samples do not. α-Synuclein oligomers also upregulate lysosomal-associated membrane protein-2A (LAMP-2A), a protein critical to chaperone-mediated autophagy. A broad search of existing drug candidates revealed that antagonists of the sigma-2 receptor complex were the most effective at blocking α-synuclein oligomer-induced trafficking deficits and LAMP-2A upregulation. These drug candidates may represent a novel therapeutic approach against Parkinson’s neuronal dysfunction and neurodegenerative disorders caused by α-synuclein oligomer-mediated toxicity.
1 INTRODUCTION
Parkinson’s disease (PD) is a neurodegenerative disorder characterized by dysfunction in motor control, diminished autonomic functions, and non-motor symptoms including cognitive loss (Aarsland et al., 2017; Mhyre et al., 2012). The hallmark histopathology that defines PD is intracytoplasmic inclusions called Lewy bodies, which contain high concentrations of the protein α-synuclein in a predominantly beta sheet fibrillar conformation (Spillantini et al., 1997). α-Synuclein is a 140 amino acid protein found in presynaptic terminals throughout the brain that has a role in controlling the movement of presynaptic vesicles and their fusion with synaptic membranes (Burré et al., 2014; Diao et al., 2013; Meade et al., 2019). In aging and disease, however, cumulative insults such as fatty acid lipid binding (Karube et al., 2008; Narayanan & Scarlata, 2001; Perrin et al., 2001), metal ions (Deas et al., 2016), oxidative stress (Esteves et al., 2009), acidosis (Meade et al., 2019), and endoplasmic reticulum (ER) stress (Jiang et al., 2010; Scheper & Hoozemans, 2015) can modulate the structure and form of endogenous α-synuclein, resulting in aggregated species such as fibrils and oligomers, which are associated with Parkinson’s pathology (Bernal-Conde et al., 2020; Meade et al., 2019; Roberts et al., 2015; Wong & Krainc, 2017). Additionally, post-translational modifications of α-synuclein identified in the brains of individuals with PD, dementia with Lewy bodies, or Alzheimer’s disease accelerate the aggregation of α-synuclein into cytotoxic soluble oligomers (Barrett & Greenamyre, 2015; Luth et al., 2015; Meade et al., 2019; Paleologou et al., 2009; Tsigelny et al., 2008).
α-Synuclein oligomers specifically, not the monomeric or fibril forms of α-synuclein peptides, have been found to disrupt intracellular trafficking (Auluck et al., 2010; Chai et al., 2013; Hunn et al., 2015; Jang et al., 2010), disrupt normal calcineurin function (Martin et al., 2012), increase intracellular calcium levels (Bernal-Conde et al., 2020; Martin et al., 2012), halt normal autophagy (Martinez-Vicente et al., 2008; Wang et al., 2016), and cause synapse dysfunction and loss (Choi et al., 2015; Diógenes et al., 2012; Scott et al., 2010). The transsynaptic spread of extracellular α-synuclein oligomers is hypothesized to underlie disease progression and correlates with Braak staging of PD (Hassink et al., 2018; Henderson et al., 2019) as well as Lewy body and synaptic pathology in neurons (Hansen & Li, 2012).
Currently there are no effective disease modifying therapeutics for PD and related synucleinopathies such as multiple system atrophy and dementia with Lewy bodies. Therapeutics that can effectively stop oligomer-induced toxicity have the potential to treat the motor and cognitive symptoms resulting from synaptic dysfunction and prevent the spread of oligomer-induced pathology during disease progression. Our goal was to identify anti-α-synuclein oligomer drug candidates by screening compounds for the ability to rescue α-synuclein oligomer-induced deficits in the target population: primary neurons.
We identified recombinant full-length α-synuclein protein oligomer preparations suitable for screening compound libraries that replicate the toxic effects of Parkinson’s patient brain-derived oligomers, using assays that measure two key aspects of cellular function known to be disrupted by α-synuclein oligomers: intracellular lipid vesicle trafficking (Izzo, Staniszewski, et al., 2014) and chaperone-mediated autophagy.
Treatment of mature primary hippocampal/cortical neuronal and glial cultures (21 days in vitro; DIV) with recombinant α-synuclein oligomers as well as α-synuclein oligomer species isolated from brain samples from individuals with PD, but not non-PD age-matched control individuals, resulted in lipid vesicle trafficking deficits. Treatment of neuronal cultures with recombinant α-synuclein oligomers also upregulated the expression of lysosomal-associated membrane protein-2A (LAMP-2A), a protein critically required for chaperone-mediated autophagy. This is the first report demonstrating that recombinant α-synuclein oligomers have a similar functional impact as PD patient brain-derived α-synuclein oligomers.
We then screened several libraries of small molecule compounds, including the NIH Clinical Collection to identify compounds capable of blocking recombinant α-synuclein oligomer-induced lipid vesicle trafficking deficits. Unexpectedly, the most effective compounds were selective sigma-2 receptor allosteric antagonists, which blocked these deficits in a dose-dependent manner. These compounds also blocked recombinant α-synuclein oligomer-induced LAMP-2A upregulation. Molecular interactions between sigma-2 receptor component proteins progesterone receptor membrane component 1(PGRMC1) and transmembrane protein 97 (TMEM97), α-synuclein, and proteins that control vesicular tracking and autophagy (such as LC3B) may form the basis for these observations. Importantly, and for the first time, these data indicate that small molecule selective sigma-2 receptor complex antagonists can impact a critical modulator in the α-synuclein signaling cascade and stop oligomer-induced deficits. Inhibitors that modulate sigma-2 receptors may be therapeutic against oligomeric α-synuclein-induced neuronal dysfunction in PD and other α-synucleinopathies.
…
3 RESULTS
3.1 α-Synuclein oligomer exposure resulted in membrane trafficking deficits in cultured primary neurons
We examined α-synuclein oligomer effects in rat primary neurons, grown for 21 DIV, which contain a mixture of both MAP2-positive neurons and various glial cell subtypes (Izzo, Staniszewski, et al., 2014) and express the full complement of synaptic proteins characteristic of neurons in the mature brain (Torre & Nicholls, 1998).
We and others have previously reported that the MTT assay can be used to measure changes in membrane trafficking rates induced by pathogenic oligomers (Hong et al., 2007; Izzo, Staniszewski, et al., 2014; Kreutzmann et al., 2010; Liu & Schubert, 1997). Significant increases in the rate of exocytosis of intracellular vesicles caused by pathogenic Aβ oligomers were observed using this assay, and the increased exocytosis rate is associated with a loss of synapses in neurons (Izzo, Xu, et al., 2014). Disruption of membrane trafficking in neurodegenerative diseases leads to the loss of neuronal function and subsequent loss of neurons (Hunn et al., 2015; Tong et al., 2004).
In the MTT assay, yellow tetrazolium salts are endocytosed by cells and reduced to insoluble purple formazan in the endosomal pathway. Following exocytosis, the insoluble formazan precipitates in aqueous media, appearing as needle-shaped crystals on the plasma membrane. Here we show that at 60 min after MTT exposure, reduced cargo dye (formazan) was visualized within intracellular vesicles in vehicle-treated cultures (Figure 1a), while cultures treated with α-synuclein oligomer for 24 hr exhibited predominantly exocytosed dye crystals (Figure 1b). This indicates that α-synuclein oligomers accelerate the rate of exocytosis. Assaying membrane trafficking as a function of α-synuclein concentration, we found that α-synuclein oligomers and high concentrations of α-synuclein monomer caused dose-dependent membrane trafficking deficits by increasing the rate of exocytosis in primary neurons (Figure 1c, wild-type α-synuclein oligomers (half maximal effective concentration (EC50) = 212 nM) and α-synuclein monomer (EC50 = 938 nM). Together, these data demonstrate that α-synuclein oligomers caused significant trafficking deficits when compared with vehicle at all concentrations >30 nM (p < 0.0500, Student’s t test, n = 8), and α-synuclein monomer caused deficits at concentrations >250 nM (p < 0.0500, Student’s t test, n = 8) (Figure 1c, f = 6.821 (1, 792), n = 8, p < 0.0010). Side by side comparison of oligomeric and monomeric preparations of recombinant full-length α-synuclein in western blots (Figure 2a) revealed that most of the protein remained in monomeric form (~17 kDa) in both preparations, and both preparations contain higher weight oligomers (>30 kDa) as well. The oligomeric preparation, however, contained a higher concentration of these >30 kDa species compared with the monomeric preparation. As is typically the case for a variety of proteins, only a small percentage of the recombinant monomer protein oligomerizes during the incubation process; most of it remains in the monomeric form. Similarly, for aggregation-prone proteins, freshly prepared monomer can also rapidly form low concentrations of oligomers, especially under denaturing gel chromatography conditions. This widely recognized phenomenon is also observed when oligomers are made from other synthetic or recombinant proteins, such as Aβ 1-42 (Izzo, Staniszewski, et al., 2014). The functional impact of oligomers that do form is profound, however; despite this small increase in α-synuclein oligomer concentration, recombinant oligomers are much more potent at inhibiting intracellular lipid vesicle trafficking than monomer, corresponding to a measurable fourfold difference in EC50 potency in the trafficking assay (Figure 1c).
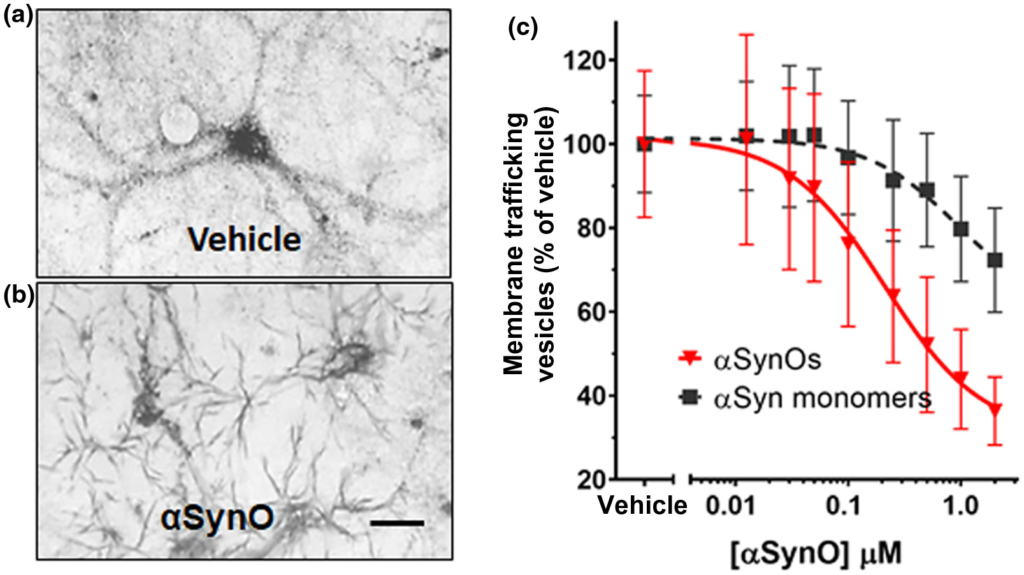
α-synuclein oligomers cause trafficking deficits in vitro.
Intracellular vesicles were labeled with reduced cargo dye (formazan) in vehicle-treated cultures (a). In contrast, reduced formazan was trafficked out of the cell more rapidly (b) following addition of recombinant α-synuclein oligomer (1 µM, total α-synuclein concentration). Size bar = 20 microns. The EC50 was calculated by determining the percentage of dye in vesicles as a function of α-synuclein concentration. α-synuclein oligomers (red triangles) dose-dependently induced deficits in vesicle trafficking (EC50 = 212 nM) (c), while α-synuclein monomer only induced trafficking deficits at concentrations >1.0 µM (c, black squares; EC50 = 938 nM). Representative images (a and b) were taken 60 min following exposure to MTT formazan. Data points represent means ± SD for three replicate experiments from separate cell culture preparations [Color figure can be viewed at wileyonlinelibrary.com]
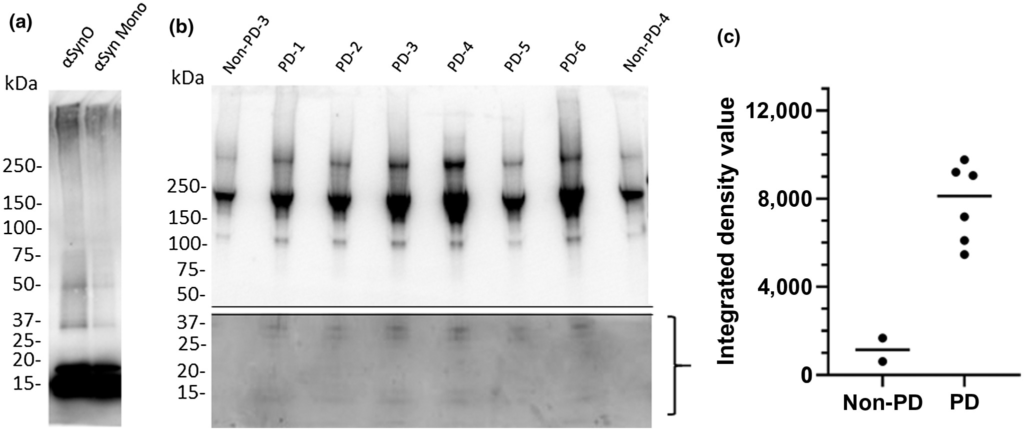
Comparison of recombinant α-synuclein oligomers with α-synuclein isolated from PD patient brain samples.
Denatured western blot comparing recombinant α-synuclein oligomer (αsynO) with α-synuclein monomer (αsyn Mono) labeled with α-synuclein antibody (syn211) reveals that both preparations contain monomer (~17 kDa) and higher weight oligomers (>30 kDa), however, the oligomeric preparation contains a higher concentration of these >30 kDa species compared with the monomeric preparation (a). A total of 1 µg of synthetic α-synuclein protein was loaded in each lane in (a). Denatured western blots of immunoprecipitated PD patient frontal cortex samples demonstrated heterogeneous populations of α-synuclein assemblies (b). Western blots of α-synuclein immunoprecipitated from six different PD patients (lanes 2–7) and two non-PD control subjects (lanes 1 and 8) exhibit major bands >100 kDa. Using a longer exposure time, a 36 kDa doublet band can be observed in the bottom half of the blot in PD patient samples (lanes 2–7) that is absent in non-PD controls (lanes 1 and 8, quantified in c), corresponding with α-synuclein oligomeric species (Ardah et al., 2014; Tsika et al., 2010; Winner et al., 2011); full gel is shown in Figure S1). (c) Measurement of integrated gel band intensity (integrated density value, IDV) in the bracketed range shown in b using FluoroChem reveals substantial differences in low molecular weight range oligomer concentrations between PD and non-PD brains
3.2 Recombinant α-synuclein oligomer and PD patient-derived α-synuclein samples contained similar low molecular weight species that were absent in non-PD control samples
Previous studies indicate that extreme non-physiological conditions, such as high detergent and temperature, are required to isolate appreciable concentrations of α-synuclein from postmortem PD-patient brain tissue (Deramecourt et al., 2006; Garcia-Esparcia et al., 2015; Kellie et al., 2014; Paleologou et al., 2009). To avoid such conditions, we explored a number of syn211 antibody-mediated immunoprecipitation methods to maximize the recovery of α-synuclein protein from PD patient postmortem brain tissue, and chose elution with 6 M guanidine HCl as the optimal condition, balancing physiological relevance and recovery concentrations.
We compared the protein size (molecular weight) ranges of PD patient brain-derived α-synuclein species and recombinant α-synuclein oligomer via western blot under nondenaturing conditions using antibody syn211 (Figure 2). Freshly prepared α-synuclein oligomer consisted of protein sizes ranging from 17 to 100 kDa with notable low molecular weight bands at 36 and 17 kDa (Figure 2a). These band sizes are consistent with the α-synuclein oligomer size range previously reported (Ardah et al., 2014; Luk et al., 2009; Recasens et al., 2014; Tong et al., 2010; Tsika et al., 2010; Winner et al., 2011). Similar protein size ranges were detected in PD patient brain-derived α-synuclein, but notably, low molecular weight bands (17 kDa, corresponding to monomer and 36 kDa, corresponding to dimer) were not present in non-PD control subjects (Figure 2b). This result is in agreement with previous reports stating that the α-synuclein dimer, which has an important role in α-synuclein oligomer aggregation and PD pathology (Medeiros et al., 2017; Roostaee et al., 2013) is present at higher levels in PD patients than non-PD controls (Papagiannakis et al., 2018). Additional high molecular weight bands (>75 kDa) were consistently observed, but to variable extents, in both PD patients and non-PD control subjects (Figure 2b). Density quantification indicates greater protein density in 10–36 kDa bands in tissue from PD patients relative to non-PD patients (Figure 2c).
3.3 α-Synuclein oligomers isolated from PD patient brains and recombinant α-synuclein oligomers caused similar lipid vesicle trafficking deficits in vitro
We compared the effects of PD patient brain-derived α-synuclein and recombinant wild-type α-synuclein oligomers on membrane trafficking rates using the MTT assay (Figure 3). PD patient brain-derived α-synuclein species (Figure 3) and recombinant α-synuclein oligomers (1 µM, Figure 1) induced similar deficits in membrane trafficking rate compared with a vehicle control. In contrast, α-synuclein isolated from the brains of non-PD control subjects (Figure 3) induced no change in membrane trafficking rate compared with vehicle control. Thus, both recombinant α-synuclein oligomers and PD patient brain-derived α-synuclein species, but not α-synuclein species derived from non-PD control brains, impact cellular membrane trafficking rates. Because the only difference in α-synuclein species detected between PD and non-PD brain on western blots (Figure 2) were the higher concentration of oligomers contained in PD brains, it is likely that the impact on trafficking observed is due to oligomers.
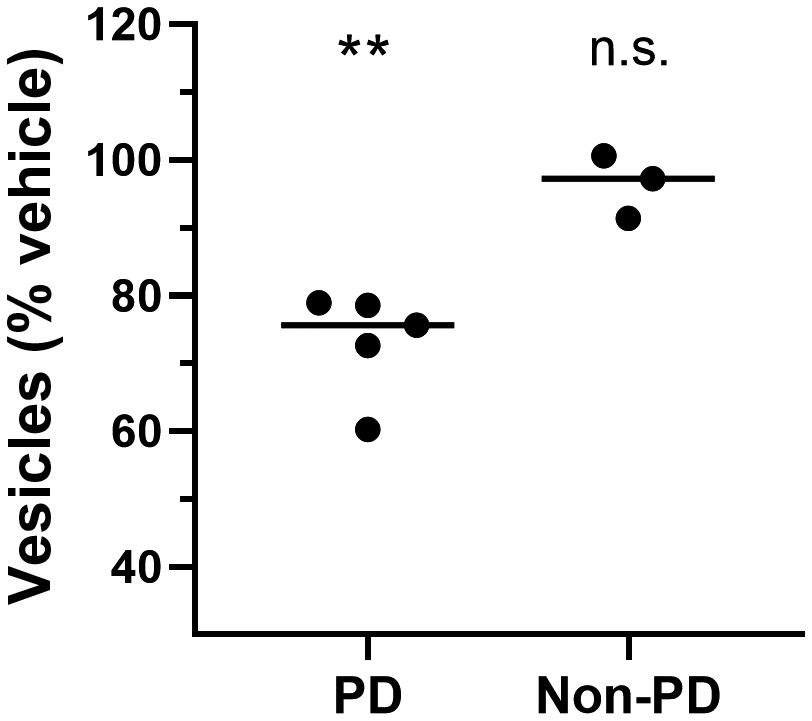
PD patient brain-derived α-synuclein oligomers induce membrane trafficking deficits in vitro.
Compared with vehicle-treated cells, α-synuclein immunoprecipitated from brain samples from multiple individuals with PD caused membrane trafficking deficits (**p ≤ 0.0100); α-synuclein from brain samples from individuals without PD did not (n.s., not significantly different from vehicle-treated cells). One-way ANOVA with Dunnett’s test for multiple comparisons. Each data point represents the mean value for an individual patient determined in quadruplicate from three separate cell preparations; undiluted samples were added to the assay wells. Black lines indicate group means
3.4 Small molecule compounds inhibited membrane trafficking deficits caused by α-synuclein oligomers in vitro
To investigate the potential of brain-penetrant small molecule therapeutic approaches, we screened for compounds capable of minimizing or eliminating α-synuclein oligomer-associated membrane trafficking deficits. The screening method was based on the MTT assay and optimized for performance in 384-well microtiter plates with automated liquid handling robotics for compound formatting and assay plate replication as previously reported (Izzo, Staniszewski, et al., 2014). We set an acceptable window for oligomer-induced effects with a 50%–80% loss of normal membrane trafficking rates due to treatment with α-synuclein oligomers, compared with vehicle treatment. We used this assay to screen the NIH Small Molecule Repository (725 compounds that have been tested in phase I-III clinical trials: full list shown in Table S1). Drugs were screened at a single assay concentration of 1 µM following the addition of α-synuclein oligomers for 24 hr, in quadruplicate wells replicated in three experiments from independent cell primary cell preparations (n = 3 experiments) with greater than 80% reversal of α-synuclein oligomer effects set as a hit. Statistical analysis indicated that these conditions had a power of 95% (GPower software, (Faul et al., 2007)) to detect false negatives. Control wells consisted of α-synuclein oligomers (1 µM) and vehicle treatment without test compounds. Of the 725 compounds, 17 compounds significantly blocked α-synuclein oligomer-induced membrane trafficking deficits. Of these, compounds with limited brain penetration or significant expected toxicity (such as oncology or anti-fungal therapeutics) were eliminated, and the resulting six hits were assayed for membrane trafficking effects across an 8-point dilution range (0.5–20 µM, Figure 4). Bioinformatics pathway analyses (MetaCore, Clarivate Analytics, Philadelphia, PA, USA) indicated that these compounds impact the retinoic acid pathway, which has previously been implicated in PD (Esteves et al., 2015; Takeda et al., 2014), confirming the disease relevance of this screening assay. Three of six compounds were weakly active (Figure 4a–c), two compounds blocked α-synuclein oligomer-induced membrane trafficking deficits at concentrations lower than 2.5 µM (lovastatin EC50 < 1 µM, carbamazepine EC50 = 2.2 µM, Figure 4d,e), and one compound (thioridazine) was cytotoxic at concentrations above 1 µM (Figure 4f).
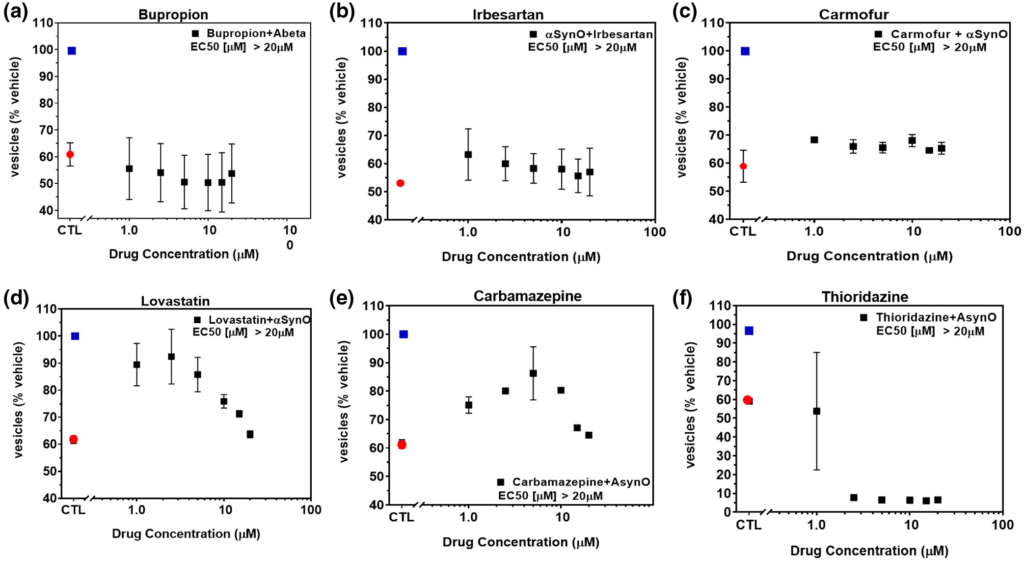
NIH Clinical Collection (NCC) small molecules inhibit α-synuclein oligomer-induced trafficking deficits.
Treatment of primary neurons with α-synuclein oligomer (1.0 μM final concentration) caused significant deficits in membrane trafficking rates (red dot) when compared with vehicle alone (blue dot, p < 0.0001, One-way ANOVA, Dunnett’s multiple comparisons). Seven hundred and twenty-five NIH small molecules were tested in single-point screens (data not shown) for their ability to block oligomer-induced deficits and a subset of hit compounds (a–f) were tested in a range of doses with select examples shown above. Addition of (a) bupropion, (b) irbesartan, or (c) carmofur weakly inhibited α-synuclein oligomer-induced trafficking deficits. (d) Lovastatin and (e) carbamazepine inhibited deficits at low concentrations but were toxic at concentrations >5 µM. (f) Thioridazine was cytotoxic at concentrations >1 µM. All data points represent means ± SD for four to six replicate experiments in separate cell culture preparations [Color figure can be viewed at wileyonlinelibrary.com]
We then screened a proprietary CogRx library of high affinity sigma-2 allosteric antagonists to identify therapeutic candidates capable of blocking oligomer-induced trafficking deficits. Several hits were obtained (Figure 5); the affinities of these compounds at the sigma-2 receptor complex are in the low nanomolar range: CT1978 (Kd = 9.2 nM), CT1970 (Kd = 1.8 nM), CT2168 (Kd = 2 nM), CT1681 (Kd = 12 nM), CT1696 (Kd = 11 nM), and CT1925 (Kd = 18 nM). When dosed against α-synuclein oligomers, all of them inhibited α-synuclein oligomer-induced trafficking deficits in a dose-dependent manner (CT1978 (EC50 = 1.48 μM), CT1970 (EC50 = 6.50 μM), CT2168 (EC50 = 1.5 μM), CT1681 (EC50 = 9.44 μM), CT1696 (EC50 = 15.53 μM), and CT1925 (EC50 = 8.95 μM) Figure 5a–f, respectively).
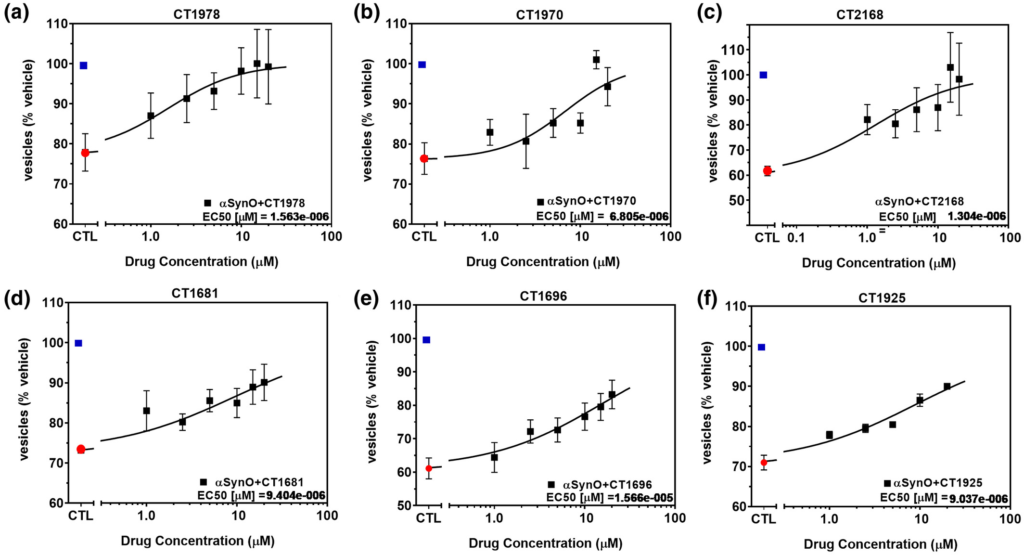
α-Synuclein oligomer-induced trafficking deficits are blocked by CogRx sigma-2 receptor antagonist small molecule therapeutic candidates.
(a–f) Treatment of primary neurons with α-synuclein oligomer (1.0 μM final concentration) caused significant deficits in membrane trafficking rates (red dot) when compared with vehicle alone (blue dot, p < 0.0001, One-way ANOVA, Dunnett’s multiple comparisons). CogRx small molecule sigma-2 antagonists blocked the effect of recombinant α-synuclein oligomer by >80%, in a dose-dependent manner (p < 0.0010 for greatest dose versus α-synuclein alone, One-way ANOVA, Dunnett’s multiple comparisons), while having no effect on membrane trafficking when dosed without the addition of α-synuclein oligomer (data not shown). All data are means ± SD for 6 replicate experiments in separate cell culture preparations [Color figure can be viewed at wileyonlinelibrary.com]
3.5 α-Synuclein oligomer-induced LAMP-2A expression increase is blocked by CogRx small molecule sigma-2 allosteric antagonist therapeutic candidates
Misfolded proteins and damaged cells are normally degraded and cleared via autophagy pathways regulated by the sigma-2 receptor complex (Mir et al., 2013); breakdown of these pathways is implicated in PD pathology (Cuervo & Wong, 2014; Xilouri et al., 2009, 2016; Yue et al., 2009), resulting in build up of α-synuclein and other proteins in neurons (Spillantini et al., 1997). Chaperone-mediated autophagy via LAMP-2A is specifically responsible for α-synuclein degradation in neurons and turnover of α-synuclein monomers (Sala et al., 2016; Vogiatzi et al., 2008) and LAMP-2A expression is dysregulated in PD (Mak et al., 2010; Orenstein et al., 2013; Sala et al., 2016). Disruption in chaperone-mediated autophagy with an inhibitor or RNA interference to LAMP-2A can lead to accumulation of α-synuclein in neuronal cells (Vogiatzi et al., 2008). Because of these links between chaperone-mediated autophagy, sigma-2 receptors, LAMP-2A, α-synuclein, and PD, we examined whether LAMP-2A expression was affected by exogenous α-synuclein oligomers in vitro, and whether sigma-2 antagonists could impact this. We quantified the intensity of LAMP-2A immunofluorescence associated with primary neuronal cells co-labeled for MAP2 and α-synuclein oligomer (Figure 6). Neurons treated with α-synuclein oligomers (0.5 μM) exhibited increased LAMP-2A immunolabeling compared with vehicle (Figure 6a,b). Sigma-2 receptor antagonist compounds CT1978 and CT2168, which actively blocked α-synuclein oligomer-induced membrane trafficking deficits (Figure 5), blocked the α-synuclein oligomer-induced increase in LAMP-2A expression (Figure 6c,d). Because the CogRx compounds are known to be specific antagonists at the sigma-2 receptor complex, these results confirm an important role for the sigma-2 receptor complex in the regulation of LAMP-2A-mediated autophagy pathways, and suggest that sigma-2 receptor antagonists may have therapeutic potential in PD.
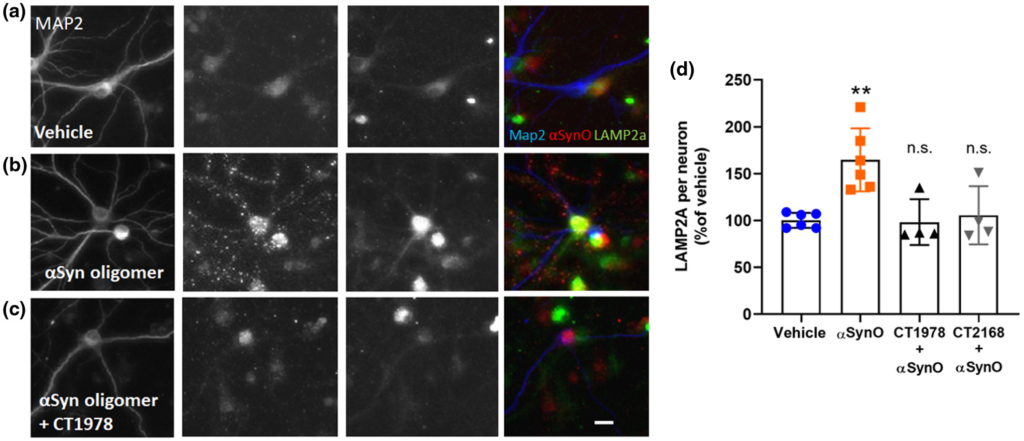
CogRx sigma-2 receptor antagonists block α-synuclein oligomer-induced autophagy dysregulation.
Neuronal cultures were treated with a low concentration (0.5 µM) of recombinant α-synuclein oligomer for 1 hr followed by CogRx compounds for 24 hr. Cells were fixed and immunolabeled to visualize MAP2-positive neuron expression of LAMP-2A and α-synuclein oligomer (antibody ASYO5). LAMP-2A expression was quantified by measuring the relative fluorescent units of puncta spots per neuron and normalized to a vehicle control. Vehicle wells demonstrated endogenous expression of LAMP-2A (a). α-Synuclein oligomers exhibit punctate distribution on neurons and increased LAMP-2A expression by > 75% (b). Treatment with CogRx compounds CT1978 (representative image, c) and CT2168 decreased α-synuclein oligomer (α-SynO) puncta intensity and LAMP-2A puncta count per neuron, more closely resembling vehicle control wells (d). Data points represent means ± SD for four replicate experiments. (**p < 0.0100, ANOVA with Dunnett’s test for multiple comparisons; n.s., not significantly different compared with vehicle-treated cells.) [Color figure can be viewed at wileyonlinelibrary.com]
4 DISCUSSION
The protein α-synuclein has a crucial role in PD and related synucleinopathies. Mutations in the α-synuclein gene (SNCA) encoding mutant α-synuclein forms such as A30P and A53T lead to familial early-onset PD. Both mutant forms of α-synuclein bind more strongly (two- to sixfold) to chaperone-mediated autophagy receptor LAMP-2A than does wild-type α-synuclein, but do not translocate into the lysosomal lumen, impairing degradation of other substrates (Cuervo et al., 2004) and shifting cellular disposal pathways to upregulate secretion of protein into the extracellular space. A variety of age-related insults such as oxidative stress (Esteves et al., 2009) impact wild-type α-synuclein structure and associated function, leading to protein accumulation and subsequent oligomerization. α-Synuclein amplifies the redox consequences of mitochondrial dysfunction in dopaminergic neurons (Van Laar et al., 2020). α-Synuclein oligomers are the most toxic structural form of the protein (Karpinar et al., 2009), triggering autophagy/lysosomal dysregulation, synaptic dysfunction, mitochondrial disruption, and ER and oxidative stress, and secretion into extracellular fluid leading to transsynaptic spread and disease progression (Fields et al., 2019).
The development of novel therapeutic approaches that alleviate neuronal dysfunction and progression of PD pathology caused by α-synuclein oligomers is an urgent unmet medical need (Fields et al., 2019; Shihabuddin et al., 2018). Cellular models using disease-relevant α-synuclein oligomers that replicate patient brain-derived oligomer toxicity on target cell populations (neurons and glia) can be an effective platform for identifying potential therapeutics.
To establish such models, we began by identifying a method for generating recombinant full-length α-synuclein oligomers that produced oligomers that replicate the toxicity of patient brain-derived species. Many such methods of generating α-synuclein oligomers from wild-type or modified protein have been published (Benner et al., 2008; Choi et al., 2013; Danzer et al., 2007; Yanying Liu et al., 2011; Outerio et al., 2009; Yu et al., 2010). Oligomers generated by seeding wild-type full length recombinant α-synuclein protein with extremely low concentrations of Aβ 1-42 oligomers (thought to act as templates to promote oligomerization of α-synuclein; Mandal et al., 2006; Martin et al., 2012; Masliah et al., 2001; Tsigelny et al., 2008)) have been reported to cause signaling deficits at low concentrations. Here for the first time, the effects of recombinant α-synuclein oligomers made with this method were compared with Parkinson’s patient brain-derived α-synuclein oligomer species effects on neurons and glia in primary culture. Both oligomer preparations disrupted normal membrane trafficking in a similar manner, whereas oligomers isolated from non-PD age-matched control brains with identical methods did not. This suggests that recombinant α-synuclein oligomers made using this method are disease relevant and appropriate for use in compound screening models of the disease process in vitro, with the much less readily available patient brain-derived oligomers used to confirm results obtained with recombinant oligomers.
Comparison of recombinant α-synuclein oligomers with human-derived α-synuclein species using western blot revealed low molecular weight species in both the recombinant α-synuclein oligomer and PD patient brain-derived α-synuclein samples, but not non-PD control samples. Consistent with previous reports, these low molecular weight α-synuclein oligomeric species potently induce changes in trafficking and autophagy consistent with disease pathology (Tsika et al., 2010; Winner et al., 2011). Similarly, low molecular weight α-synuclein species have been shown to disrupt synaptic vesicle fusion and transmission (Medeiros et al., 2017). Notably, the human brain-derived α-synuclein preparation described here was shown for the first time to yield α-synuclein protein species that caused trafficking deficits. Future studies will be required to characterize recombinant and PD patient brain-derived oligomers in more detail with larger numbers of patient brain samples. Evidence indicates that soluble extracellular α-synuclein oligomers can be transmitted between neighboring cells, which is thought to be the mechanism of the spread of disease pathology (Domert et al., 2016). Addition of exogenous recombinant α-synuclein oligomers to primary neurons in culture may model this aspect of PD pathology in addition to intracellular effects. α-Synuclein monomer had reduced effects on membrane trafficking deficits when compared with oligomers, an important functional difference between the two structural forms that may provide insight into early stages of disease development.
Cellular assays that measure processes disrupted in disease in primary neurons are also important for translational modeling of disease. We chose to use assays that measure two key aspects of neuronal function known to be disrupted by α-synuclein oligomers: intracellular lipid vesicle trafficking (Izzo, Staniszewski, et al., 2014) and chaperone-mediated autophagy. Intracellular lipid vesicle trafficking-dependent processes are disrupted in PD and are thought to underlie cognitive and motor deficits (Alvarez-Erviti et al., 2011; Ben Gedalya et al., 2009; Chai et al., 2013; Jang et al., 2010; Lee et al., 2005), stemming from α-synuclein’s central role in normal soluble N-ethylmaleimide-sensitive fusion attachment protein receptor (SNARE)-mediated vesicle trafficking and regulation of synaptic vesicle exocytosis (Burré et al., 2014; Diao et al., 2013; Hunn et al., 2015). We used a previously established assay of intracellular lipid vesicle trafficking rate (Izzo, Staniszewski, et al., 2014; Izzo, Xu, et al., 2014) to model this process; this assay has been used to identify disease-modifying Alzheimer’s therapeutic candidates.
Chaperone-mediated autophagy is one of several processes that degrade and remove altered proteins in response to cell stress or toxic material (Martinez-Vicente et al., 2008; Vogiatzi et al., 2008), and regulates amino acid recycling and protein quality control (Martinez-Vicente & Cuervo, 2007; Sala et al., 2016). The lysosomal receptor LAMP-2A controls the translocation of substrates to the lysosomal lumen, and the rate of chaperone-mediated autophagy is regulated by the assembly and disassembly of the LAMP-2A translocation complex (Sala et al., 2016). LAMP-2A function is pivotal to PD disease progression (Cuervo & Wong, 2014; Mak et al., 2010; Martinez-Vicente & Cuervo, 2007; Sala et al., 2016; Vogiatzi et al., 2008). Disruption of chaperone-mediated autophagy with a LAMP-2A inhibitor can lead to accumulation of α-synuclein in neuronal cells (Vogiatzi et al., 2008). α-Synuclein and LAMP-2A co-immunoprecipitate in culture (Vogiatzi et al., 2008) and overexpressed wild-type or mutant α-synuclein binds to LAMP-2A and disrupts internalization and degradation (Cuervo et al., 2004; Fonseca et al., 2015; Roberts & Brown, 2015; Sala et al., 2016). These and other data lead to the hypothesis that increasing LAMP-2A levels is a compensatory mechanism to increase the autophagic response to toxic α-synuclein oligomers (Mak et al., 2010; Orenstein et al., 2013). In agreement with this, the present results indicate that neurons treated with recombinant α-synuclein oligomers significantly upregulate LAMP-2A protein expression.
To identify small molecule drug candidates capable of blocking recombinant α-synuclein oligomer-induced toxicity, we screened several compound libraries (including the NIH Clinical Collection) in the previously established lipid vesicle trafficking rate assay. Unexpectedly, potent and specific sigma-2 receptor antagonists (Izzo, Staniszewski, et al., 2014; Izzo, Xu, et al., 2014) most effectively dose-dependently blocked α-synuclein oligomer-induced deficits in membrane trafficking, and also inhibited LAMP-2A upregulation by oligomers. The effective inhibitor compounds identified within the NIH Clinical Collection partially reduced the effects of α-synuclein oligomers at low concentrations, but then were cytotoxic themselves at higher concentrations. In contrast, the small molecule sigma-2-specific CogRx inhibitors were effective at low concentrations and did not exhibit cytotoxicity at similar higher concentrations. This is the first demonstration that allosteric antagonists of the sigma-2 receptor complex have anti-α-synuclein oligomer effects.
One possible molecular basis for these observations is the direct role that sigma-2 receptor complex component proteins PGRMC1 (Riad et al., 2018, 2020; Xu et al., 2011) and TMEM97 (Alon et al., 2017) have in both intracellular lipid vesicle trafficking and autophagy. PGRMC1 contains several immunoreceptor tyrosine-based activation motif consensus sequences and binds directly to several vesicle trafficking regulatory proteins including N-ethylmaleimide-sensitive factor, microtubule-associated proteins 1A/1B light chain 3B, ultra-violet radiation resistance-associated gene (UVRAG), unc-51-like autophagy activating kinase 2, autophagy-related 5, and RB1-inducible coiled-coil protein 1 (Behrends et al., 2010; Mir et al., 2013). PGRMC1 regulates trafficking of a wide variety of transmembrane receptors between subcellular compartments and the plasma membrane and is required to stabilize these receptors in the plasma membrane including epidermal growth factor receptor (Ahmed et al., 2010), membrane progesterone receptor α (Thomas et al., 2014), glucagon-like peptide receptor type 1 (Zhang et al., 2014), netrin receptor (UNC-40/deleted in colorectal cancer; UNC-40/DCC) (Runko & Kaprielian, 2004), and insulin receptors (Hampton et al., 2018); reduction of PGRMC1 levels via genetic knockdown results in internalization of these receptors. Direct binding of PGRMC1 to MAP1LC3B and UVRAG is required for normal autophagy (Mir et al., 2013). Both lipid vesicle formation/trafficking and autophagy place large demands on cellular lipid membrane synthesis and degradation, and both PGRMC1 and TMEM97 directly regulate cholesterol synthesis. Most of TMEM97’s sequence (aa 10-158) is an Expera domain, likely to possess direct sterol isomerase catalytic activity (Sanchez-Pulido & Ponting, 2014). TMEM97 itself binds directly to NPC1 (the protein that is mutated in the sphingolipid storage disorder Niemann–Pick disease); reduction of TMEM97 upregulates NPC1 and restores cholesterol synthesis and trafficking in Niemann–Pick cells (Ebrahimi-Fakhari et al., 2015). PGRMC1 interacts with Nr4a1 (an immediate early gene required for the induction of several genes encoding steroidogenic enzymes) to regulate neurosteroid synthesis or signaling in neuronal cell lines (Intlekofer et al., 2019) a possible protective mechanism in response to neuroinflammatory signaling. In non-neuronal cells, PGRMC1 regulates cholesterol synthesis via two direct binding interactions; to P450 proteins (Rohe et al., 2009) and to Insig and SCAP (Hughes et al., 2007; Suchanek et al., 2005); under low cholesterol conditions, Insig/SCAP dissociates from PGRMC1 and translocates to the nucleus where they mediate SRE-related gene transcription). Finally, both PGRMC1 and TMEM97 bind directly to the low-density lipoprotein receptor (LDL); this complex binds LDL, apoE, and monomeric and oligomeric Aβ and contributes to their internalization into neurons (Riad et al., 2018).
α-Synuclein oligomers bind directly to PGRMC1 in brain synaptosomes (Betzer et al., 2015), and α-synuclein binds directly to LAMP-2A in the process of chaperone-mediated autophagy (Schneider & Cuervo, 2014; Vogiatzi et al., 2008). α-Synuclein oligomers interact with and disrupt cholesterol-rich lipid membranes and disrupt related downstream signaling effects, including the process of autophagy (Galvagnion, 2017; Galvagnion et al., 2016; Plotegher et al., 2017; Stefanovic et al., 2014; Van Maarschalkerweerd et al., 2015). Thus, allosteric antagonists of the sigma-2 receptor proteins may alleviate the negative impacts of α-synuclein oligomers through a variety of mechanisms.
The present results are the first demonstration that PD patient brain-derived α-synuclein oligomers and recombinant α-synuclein oligomers both inhibit intracellular lipid vesicle trafficking, providing strong support for the disease relevance of recombinant α-synuclein oligomers made using the described methods and the translational applicability of using recombinant oligomers in screens to develop drug candidates. These results are also the first demonstration that sigma-2 allosteric antagonists effectively reduced both the α-synuclein oligomer-induced lipid vesicle trafficking deficit and upregulation of LAMP-2A. The sigma-2 receptor complex proteins regulate multiple cellular damage response pathways in which α-synuclein directly participates, and that are impacted in PD. These results suggest that the ability of sigma-2 allosteric antagonists to block α-synuclein oligomer toxicity may result from the role of sigma-2 receptor proteins in regulating intracellular lipid vesicle trafficking, autophagy, and cholesterol metabolism. These data support the hypothesis that targeting the sigma-2 receptor complex with brain-penetrant small molecule antagonists represents a tractable therapeutic approach to alleviating α-synuclein-induced pathology in PD and other α-synucleinopathies.

