FMR1 deletion in rats induces hyperactivity with no changes in striatal dopamine transporter availability
By Annunziata D’Elia, Sara Schiavi, Antonia Manduca, Alessandro Rava, Valeria Buzzelli, Fabrizio Ascone, Tiziana Orsini, Sabrina Putti, Andrea Soluri, Filippo Galli, Alessandro Soluri, Maurizio Mattei, Rosella Cicconi, Roberto Massari, and Viviana Trezza
Excerpt from the article published in Scientific Reports 12, 22535, 29 December 2022. DOI: https://doi.org/10.1038/s41598-022-26986-2
Editor’s Highlights
- Fragile X syndrome (FXS) is commonly caused by a trinucleotide repeat expansion of CGG in the promoter region of Fragile X Messenger Ribonucleoprotein 1 (FMR1) gene, leading to methylation, transcriptional silencing and to the absence or deficiency of FMRP.
- Fmr1-Δexon 8 rats have been recently validated as a genetic animal model of autism spectrum disorder (ASD) and rat model of FXS.
- Fmr1 Δexon 8 rats displayed hyperactivity in the open field test in the absence of repetitive behaviors in the hole board test
- Behavioral alterations were not associated with changes in striatal dopamine transporter (DAT) availability as assessed by non-invasive in vivo SPECT and Western blot analyses.
Abstract
Autism Spectrum Disorder (ASD) is a pervasive neurodevelopmental disorder emerging in early life characterized by impairments in social interaction, poor verbal and non-verbal communication, and repetitive patterns of behaviors. Among the best-known genetic risk factors for ASD, there are mutations causing the loss of the Fragile X Messenger Ribonucleoprotein 1 (FMRP) leading to Fragile X syndrome (FXS), a common form of inherited intellectual disability and the leading monogenic cause of ASD. Being a pivotal regulator of motor activity, motivation, attention, and reward processing, dopaminergic neurotransmission has a key role in several neuropsychiatric disorders, including ASD. Fmr1 Δexon 8 rats have been validated as a genetic model of ASD based on FMR1 deletion, and they are also a rat model of FXS. Here, we performed behavioral, biochemical and in vivo SPECT neuroimaging experiments to investigate whether Fmr1 Δexon 8 rats display ASD-like repetitive behaviors associated with changes in striatal dopamine transporter (DAT) availability assessed through in vivo SPECT neuroimaging. At the behavioral level, Fmr1 Δexon 8 rats displayed hyperactivity in the open field test in the absence of repetitive behaviors in the hole board test. However, these behavioral alterations were not associated with changes in striatal DAT availability as assessed by non-invasive in vivo SPECT and Western blot analyses.
Introduction
The 5th Edition of the Diagnostic and Statistical Manual of Mental Disorders1 defines autism spectrum disorder (ASD) as a neurodevelopmental disorder characterized by persistent deficits in social communication and interaction and restricted-repetitive patterns of behavior, interests or activities. Fragile X syndrome (FXS) is a common form of inherited intellectual disability (ID) and the leading monogenic cause of ASD2,3. It is most commonly caused by a trinucleotide repeat expansion of CGG in the promoter region of Fragile X Messenger Ribonucleoprotein 1 (FMR1) gene, leading to methylation, transcriptional silencing and to the absence or deficiency of FMRP. FMRP is an RNA binding protein with a key role in the translational control of several mRNAs, many of which are involved in the maintenance and development of synaptic function and plasticity4. Therefore, in the absence of this protein, deregulation of translation, transport, and mRNA stability affects multiple neuronal pathways, generating the characteristic phenotype of FXS patients. Approximately 30% of patients with FXS meet the full diagnostic criteria for ASD5, and over 90% of individuals with FXS display some ASD symptoms6, including cognitive deficits, social dysfunctions, mood lability, hyperactivity, altered sensory processing and seizures.
Dopaminergic neurotransmission is a critical regulator of motor function, reward, motivation, attention and learning7,8,9,10. Dysregulations of the brain dopaminergic system have been implicated in a number of neurological and neuropsychiatric disorders, including ASD11,12,13. Interestingly, the dopamine (DA) hypothesis of ASD claims that dysfunctions in the midbrain dopaminergic system could contribute to autistic-like behaviors14: thus, the social deficits observed in ASD could reflect a mesocorticolimbic circuit dysfunction, while the repetitive/stereotyped behaviors could arise from a dysfunction of the nigrostriatal pathway15. Dopaminergic projections originating from the substantia nigra and ventral tegmental area terminate in the striatum, where they regulate motor function and overall activity and influence thalamocortical signaling16. The striatal complex of basal ganglia comprises two functionally distinct districts, the dorsal and ventral striatum, with the former mainly involved in the control of motor activities together with procedural memory storage, and the latter principally mediating motivation, reward, and emotion17. Due to the prominent role of the striatum in sensorimotor function (e.g., locomotor control and habit formation), associative tasks (e.g., goal-directed behavior) and motivational behavior18,19,20 and its abundance in DA projections21, it is possible that aberrant DA striatal signaling could promote the stereotyped and perseverative patterns of behaviors typical of ASD22,23.
The DA transporter (DAT) plays a fundamental role in maintaining optimal DA signaling, since it regulates the temporal and spatial availability of DA24 by rapidly clearing released DA from the synapse. Interestingly, DAT gene mutations have been linked to ASD12, and altered DAT expression in rodents has been correlated to hyperactivity and repetitive behaviors25,26, which are hallmarks of ASD27,28. Though these studies have been incremental to the field, our understanding of the impact of DAT dysregulation in the behavioral deficits typically associated with ASD and its comorbidities is still limited.
Fmr1-Δexon 8 rats have been recently validated as a genetic animal model of ASD and rat model of FXS29. Interestingly, the Fmr1-Δexon 8 rat model—generated by zinc-finger nucleases (ZFN)—results in a gene product with a loss of exon 8 which encodes a domain within the FMR1 gene that is responsible for RNA-binding, the KH1 domain. Although this animal model differs in the type of mutation from humans, this deletion is sufficient to cause FXS-like traits. In line with this, we have recently shown that Fmr1-Δexon 8 rats show cognitive, communicative and social impairments30,31, suggesting the validity of this animal model in mimicking the key behavioral deficits that characterize FXS and some of the core and comorbid features of non-syndromic ASD. Here, we investigated whether Fmr1-Δexon 8rats show altered motor function eventually associated with changes in striatal DAT availability.
During the last decade, high-resolution positron emission tomography (PET) and single-photon emission computed tomography (SPECT) scanners have been increasingly employed to study neurotransmitter transporter and receptor binding in small laboratory animals, giving new insights into the involvement of dopaminergic neurotransmission in neurological and psychiatric disorders32,33,34,35. Indeed, while other tools have been used in the preclinical field to assess DAT function, some techniques are either invasive or ex vivo techniques that do not allow longitudinal studies. Conversely, the use of a neuroimaging technique such as SPECT allows the correlation between behavioral outputs and the activation/inactivation of a specific molecular target in a given brain region. Upon injection of the radiopharmaceutical, SPECT freezes a snapshot of brain activity, which might be then recorded and analyzed. Additionally, while other techniques, such as chronoamperometry or voltammetry, may provide better temporal and spatial resolutions, SPECT provides not only quantification but also the visualization of data in their anatomical reference through its simple integration with other morphological imaging systems. Furthermore, it is a relatively simple technique that causes minimal stress to the animal by being non-invasive, and it has the potential of enabling longitudinal studies. To allow translational research, several dedicated imaging systems for small laboratory animals, such as mice and rats, have been developed over the years. Nevertheless, the extension of imaging modalities from humans to small animals faces some limitations mainly related to the differences in size and intensity of biochemical signals between the two species. For a better multidimensional understanding of complex pathophysiological phenomena, the real current challenge would be to design increasingly sensitive systems with high spatial resolution. In this context, our group has recently developed an innovative SPECT system for small organ imaging and neuroimaging that implements a super spatial resolution (SSR) method to exceed imaging capabilities achievable with traditional systems36,37,38,39. This system offers the unique opportunity to improve achievable performance with the potential to obtain higher-quality preclinical functional brain imaging39. This is achieved by moving the detector with sub-pixel shifts. In this way, the counting information of the pixel area is virtually divided into smaller areas, thus increasing the resolution of the images. The exploitation of the SSR algorithm for scintigraphic applications represents a breakthrough that might improve the performance of new generation SPECT scanners. Taking advantage of this technique, we combined behavioral and in vivo SPECT neuroimaging analyses as a preliminary investigation of the role of striatal DAT in the dysfunctional motor behavior of Fmr1-Δexon 8 rats. Exploring the neurobiological mechanisms involved in repetitive behavior and motor impairments in ASD and co-occurring conditions including FXS will improve our understanding of the pathogenesis of these developmental neuropsychiatric disorders, stimulating research on novel therapeutic approaches to these conditions.
…
Results
Behavioral studies. Stereotypic behavior and locomotor activity in Fmr1- Δexon 8 rats
Both juvenile and adult Fmr1-Δexon 8 rats did not display repetitive behaviors in the hole board test as the number of head dippings did not differ between WT and Fmr1-Δexon 8 rats (PND 35–40: t = 0.34, p = n.s, df = 34; PND 80–85: t = 0.38, p = n.s, df = 31; Fig. 2A and G). Moreover, both juvenile and adult Fmr1-Δexon 8 rats did not display repetitive behaviors in the open field test as the number of rearings (PND 35–40: t = 0.52, p = n.s, df = 14; PND 80–85: t = 0.94, p = ns, df = 18; Fig. 2 (B and H) and wall rearings (PND 35–40: t = 1.92, p = n.s, df = 14; PND 80–85: t = 0.14, p = ns, df = 18; Fig. 2C and I) was similar to the WT group.
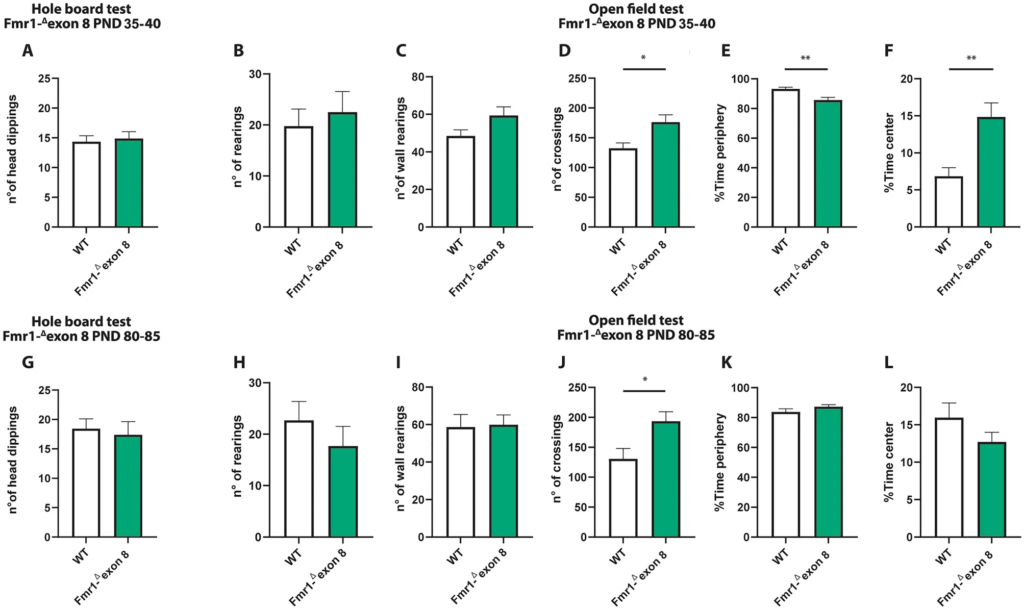
Behavioral studies: Stereotyped behavior and locomotor activity in Fmr1-Δexon 8 rats. Both juvenile (A) and adult (G) Fmr1-Δexon 8 rats did not display repetitive behaviors in the hole board test as the number of head dippings did not differ between wild-type (WT) and Fmr1-Δexon 8 rats (PND 35-40: WT = 11; Fmr1-Δexon 8 = 14; PND 80-85: WT = 18; Fmr1-Δexon 8 = 15). Moreover, juvenile and adult Fmr1-Δexon 8 rats did not display repetitive behaviors in the open field test as their number of rearings (B and H) and wall rearings (C and I) was similar to WT animals. Conversely, Fmr1-Δexon 8 rats displayed hyperactivity in the open field test as expressed in the increased number of crossings when compared to WT controls (D and J). Moreover, juvenile Fmr1-Δexon 8 rats spent less time in the periphery of the open field (E) and longer time in the center of the arena (F) as compared to WT rats. These differences were not observed at adulthood (K and L, respectively) (PND 35-40: WT = 8; Fmr1-Δexon 8 = 8; PND 80-85: WT = 10; Fmr1-Δexon 8 = 10). Data represents means ± SEM. *p < 0.05, **p < 0.01 versus WT group (Student’s t-test).
Conversely, Fmr1-Δexon 8 rats displayed hyperactivity in the open field test as they presented an increased number of crossings when compared to their WT controls (PND 35–40: t = 2.92, p < 0.05, df = 14; PND 80–85: t = 2.69, p < 0.05, df = 18; Fig. 2D and J). Moreover, only juvenile Fmr1-Δexon 8 rats spent less time in the periphery of the open field as compared to WT rats (PND 35–40: t = 3.47, p < 0.01, df = 14; PND 80–85 t = 1.40, p = n.s., df = 18; Fig. 2E and K), and longer time in the central part of the arena (PND 35–40: t = 3.56, p < 0.01, df = 14; PND 80–85: t = 1.40, p = n.s., df = 18; Fig. 2F and L), suggesting a reduced thigmotaxis in juvenile but not adult Fmr1-Δexon 8 rats. To evaluate whether the hyperactivity displayed by Fmr1-Δexon 8rats could be related to an anxious phenotype, we also performed the elevated plus maze test across development. As a result, both juvenile and adult Fmr1-Δexon 8 rats did not display anxiety-like behaviors (Supplementary Fig. 1): for instance, no differences were found in the percentage of time spent in the open arms (PND 35–40: t = 0.03, p = n.s., df = 33; PND 80–85: t = 0.24, p = n.s., df = 34; Supplementary Fig. 1 (A and E)), in the percentage of open arm entries (PND 35–40: t = 0.43, p = n.s., df = 33; PND 80–85: t = 0.20, p = n.s., df = 34; Supplementary Fig. 1 (B and F)), and in the frequency of head-dippings (PND 35–40: t = 0.77, p = n.s., df = 33; PND 80–85: t = 0.20, p = n.s., df = 34; Supplementary Fig. 1 (C and G)). Conversely, both juvenile and adult Fmr1-Δexon 8 rats displayed hyperactivity in the elevated plus maze test as they showed an increased number of total arm entries when compared to their WT controls (PND 35–40: t = 3.64, p < 0.001, df = 33; PND 80–85: t = 2.87, p < 0.01, df = 34; Supplementary Fig. 1 (D and H)). This further confirms the hyperlocomotion displayed by Fmr1-Δexon 8 rats across development.
Neuroimaging studies
Figure 3 shows the characteristic planar images of [123I]FP-CIT uptake of two WT rats (panel A) and two Fmr1-Δexon 8 (panel B) rats, respectively. Radioactivity accumulations are clearly visible in the striatum. Moreover, the image also highlights the ROIs related to both striatum and cerebellum for each experimental group. SBRSTR analysis reveals that in the WT group the striatal specific binding ratio was 0.608 ± 0.087 (mean ± S.E.M.), whereas for the Fmr1-Δexon 8 rats the value was 0.658 ± 0.034 (mean ± S.E.M.). As a result, the unpaired t-test revealed no significant between-group differences (t = 0.539, p = 0.604, df = 8, Fig. 3C). Since the images also showed enhanced DAT levels in the midbrain, its specific binding ratio have been assessed. Namely, the midbrain specific binding ratio was 0.332 ± 0.135 (mean ± S.E.M.) in the WT group, and 0.403 ± 0.034 (mean ± S.E.M.) in Fmr1-Δexon 8 rats. As with striatal uptake, the unpaired t-test revealed no significant between-group differences (t = 0.716, p = 0.548, df = 2). An example of the characteristic distribution of DAT in the striatum in both experimental groups, WT (top) and Fmr1-Δexon 8 (bottom), is also shown through an orthogonal view of the SSR SPECT OSEM reconstruction restricted to CT brain data (Fig. 4). Moreover, the DAT binding distribution with respect to the correlated CT brain volume obtained from a 3D rendering of the dataset is shown in Fig. 5 (WT on top and Fmr1-Δexon 8on bottom images). An initial visual analysis of the images for both groups does not show a difference in the number and extent of radiopharmaceutical uptake, which is overall normal for both nigrostriatal systems. This assessment is corroborated by semi-quantitative SBRSTRanalysis, which reveals no substantial differences between the two groups.

DAT binding. The estimates for the binding potentials were evaluated through the equilibrium ratios of the distribution areas of the specifically (striatum) and non-specifically (cerebellum) bound and striatal specific binding ratios (SBRSTR) for WT and Fmr1-Δexon 8 rats. (A) [123I]FP-CIT binding to striatal DAT in two WT adult rats. (B) [123I]FP-CIT binding to striatal DAT in two Fmr1-Δexon 8 adult rats. The striatal specific DAT binding ratio is shown in (C) (WT = 5; Fmr1-Δexon 8 = 5). Data represents means ± SEM (Student’s t-test).
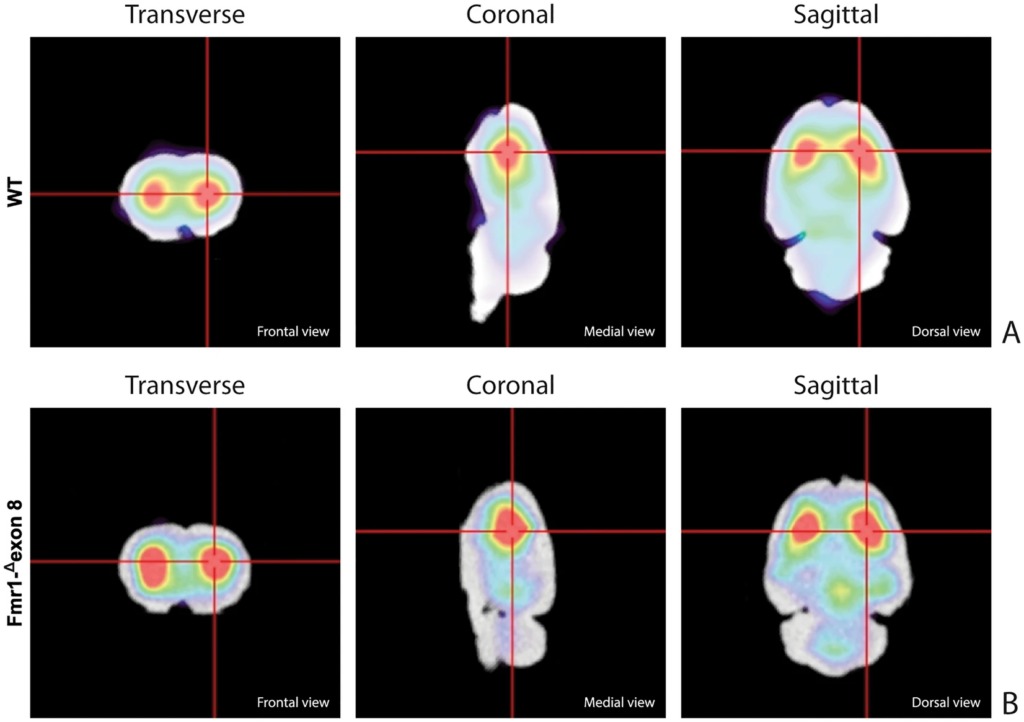
SPECT OSEM reconstruction of the distribution of [123I]FP-CIT uptake in the rat brain. (A) Orthogonal views in WT adult rats. (B) Orthogonal views in Fmr1-Δexon 8 adult rats.

Rat brain volumetric rendering applying the 2-step trans-axial SSR technique. (A) [123I]FP-CIT 3D uptake in WT adult rats. (B) [123I]FP-CIT 3D uptake in Fmr1-Δexon 8 adult rats.
Immunofluorescence and immunohistochemistry analysis
Hematoxylin and eosin (H&E) staining was performed to visualize possible gross histopathological changes in the dorsal striatum of Fmr1-Δexon 8 rats as compared to their WT controls. We found that the structure of the Fmr1-Δexon 8 and WT rat brain tissues analyzed by H&E histology (Fig. 6A and B for WT rats, D and E for Fmr1-Δexon 8 rats) was comparable at the level of the dorsal striatum (marked with a black arrow), suggesting no changes in the gross tissue organization of this brain region between Fmr1-Δexon 8 and WT rats. Moreover, immunohistochemical staining relative to DAT expression (highlighted as a dark brown dye) in the dorsal striatum (Fig. 6C for WT and F for Fmr1-Δexon 8 rats) showed no qualitative differences between genotypes as also confirmed by immunofluorescence in the striatal DA axonal projections (Fig. 6G, H and I for WT and J, K and L for Fmr1-Δexon 8rats). These findings strengthen our neuroimaging results revealing no differences in DAT between genotypes.

Histological and immunostaining assays. Microphotographs of hematoxylin and eosin staining of dorsal striatum of WT (A and B) and Fmr1-Δexon 8 (D and E) rats at lower magnification (6X, A and D) and higher magnification (40 × , B and E). Representative DAT immunohistochemistry in the dorsal striatum of adult WT (C) and Fmr1-Δexon 8 (F) rats. The dorsal striatum and surrounding corpus callosum are indicated with a black arrow and asterisks, respectively, both in histological and immunohistochemistry images. Scale-bar: 100 µm. DAT immunofluorescence and DAPI nuclear staining in the dorsal striatum of adult WT (G–I) and Fmr1-Δexon 8 (J–L). Red DAT (G and J); blue DAPI (H and K); merge (I and L). Scale-bar: 100 µm.
Western blot analysis
To estimate the protein expression level of DAT in the striatum of Fmr1-Δexon 8 rats, we performed a western blotting analysis in both the dorsal (Fig. 7A and B) and ventral striatum (Fig. 7C and D). In line with SPECT and immunohistochemical findings, the DAT protein levels in both the dorsal (t = 1.02, df = 6, p = n.s.) and ventral (t = 0.81; df = 6; p = n.s.) striatum did not differ between WT and Fmr1-Δexon 8 animals. Overall, these data confirm that FMRP deficiency does not induce a dysregulation in striatal DAT expression and that no regional (dorsal vs. ventral striatum) differences in DAT expression underlie the dysfunctional motor behavior displayed by Fmr1-Δexon 8 rats. Original uncropped membranes are shown in Supplementary Fig. 2 (A and B).
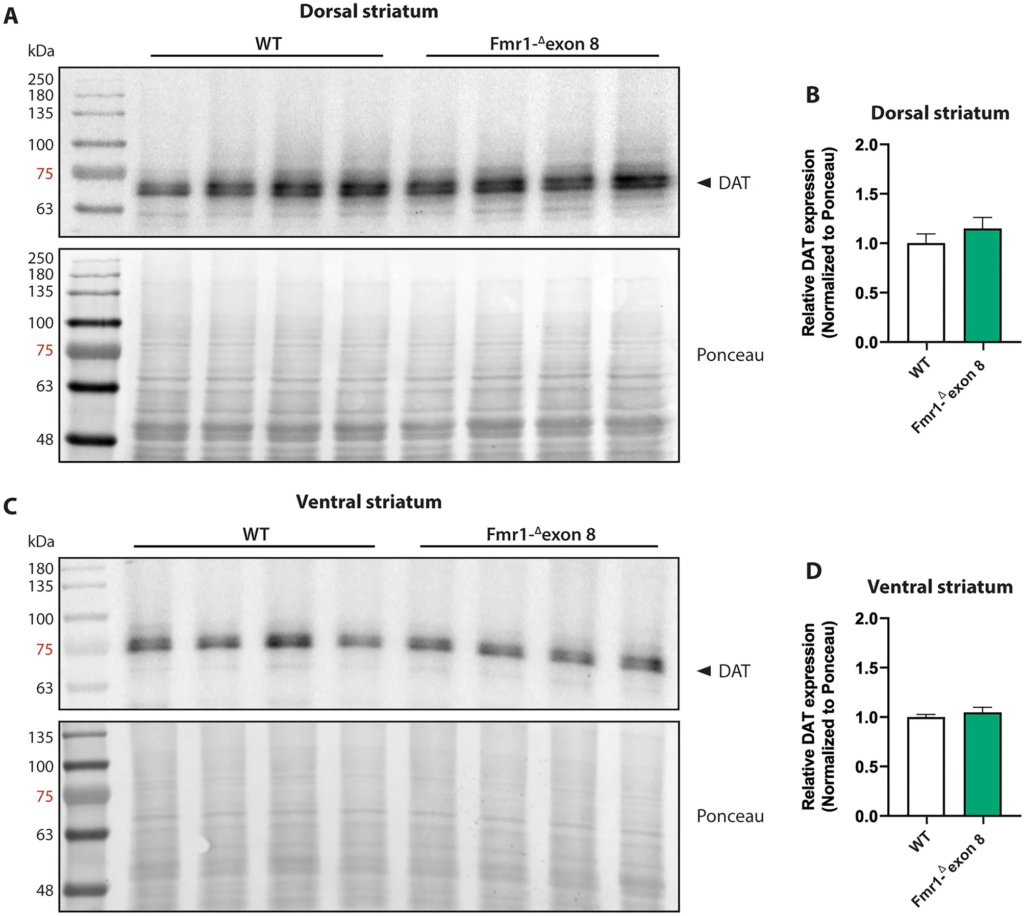
Western blot analysis. Representative western blot images of DAT in the dorsal (A) and ventral (C) striatum lysates from adult WT and Fmr1-Δexon 8 rats. Quantitative analysis of DAT protein levels in the dorsal striatum (B) and ventral striatum (D) from adult WT and Fmr1-Δexon 8 rats (WT = 4; Fmr1-Δexon 8 = 4). Densitometric values of Fmr1-Δexon 8 rats were normalized on Ponceau staining and expressed as relative fold change on WT animals. Data represents means ± SEM (Student’s t-test).
Discussion
In the present work, we combined behavioral, biochemical and in vivo neuroimaging analyses to investigate the role of striatal DAT expression in the dysfunctional motor behavior of Fmr1-Δexon 8 rats, a genetic animal model of ASD and a rat model of FXS.
Since FXS patients often show hyperactivity and repetitive patterns of behavior 3, we here tested Fmr1-Δexon 8 rats in two behavioral tasks aimed to reveal possible stereotyped/repetitive behaviors and exploratory/locomotor alterations: the hole board and the open field tests. Our results showed that both juvenile and adult Fmr1-Δexon 8 rats displayed hyperactivity in the open field test expressed as increased number of crossings while performing the task. Juvenile Fmr1-Δexon 8 rats also spent less time in the peripheral part of the open field (that is, close to the walls of the open field). To evaluate whether the hyperactivity displayed by Fmr1-Δexon 8 rats could be related to changes in anxiety-like behaviors, we also performed the elevated plus maze test. Both juvenile and adult Fmr1-Δexon 8 rats did not display anxiety-like behaviors in this task. Conversely, both juvenile and adult Fmr1-Δexon 8 rats showed an increased number of total entries in the arms of the elevated plus maze when compared to WT controls, thus confirming the hyperlocomotion displayed by Fmr1-Δexon 8 rats across development. In line with these results, the extensively characterized Fmr1-KO mouse model of FXS has been shown to display motor alterations and hyperactivity63,64,65,66,67, although normal locomotor activity has also been also reported in rat models of FXS68,69. Conversely, we found that Fmr1-Δexon 8 rats did not show stereotypic/repetitive behaviors in the hole-board test, as the number of head dippings did not differ from their WT controls. Depending on the animal strain and the behavioral task used, some studies66,70,71 but not others63,69,72 reported stereotyped behaviors in both rat and mouse models of FXS. Thus, we cannot exclude that Fmr1-Δexon 8 rats would show repetitive patterns of behavior if different tasks or experimental protocols were used.
Evidence for an involvement of dopaminergic neurotransmission in ASD arises from neuroimaging, genetic and pharmacological studies in individuals with autism but also from preclinical research performed in rodent models of ASD73. Over the years, techniques as SPECT/PET have been employed enabling imaging of the dopaminergic system in different psychiatric and neurological disorders. Such methods are often based on the assessment of DAT density as a marker for dopaminergic neuron integrity55,74,75,76. Generally, a radiolabel such as 123I-FP-CIT (DaTSCAN) is used since it is able to bind with high affinity to the presynaptic DAT located on axon terminals in the striatum. In the clinical practice, SPECT with 123I-FP-CIT can be reported as normal or abnormal through the semi-quantitative measurement of the 123I-FP-CIT signal uptake in the DAT55. Therefore, imaging with specific DA-related tracers represents a valuable tool to evaluate the status of presynaptic nigrostriatal terminals. In particular, the radiotracer DaTSCAN has become part of the diagnostic guidelines for α-synucleinopathies (e.g., Parkinsonian Syndromes, multiple system atrophy, and dementia with Lewy bodies), being approved by the most competent international authorities (i.e., FDA and EMA)77,78. From a methodological point of view, SPECT images might be exploited to determine to what extent this tracer is accumulated in the striatum compared to the background signal. As a result, reduced DAT striatal binding is therefore suggested to depict reduced DAT availability which in turn reflects striatal dopaminergic deficit79. PET imaging showed increased DAT binding in the orbitofrontal cortex of high-functioning adults with ASD80, although a SPECT study in children with autism showed no changes in DAT binding81. Interestingly, a study that examined striatal dopamine functioning during monetary incentive processing in ASD patients and controls using simultaneous PET and fMRI reported impaired phasic DA release to rewards in the striatum of patients with autism82. While these clinical findings support the involvement of functional changes in dopaminergic neurotransmission in ASD, differences in experimental procedures and heterogeneity in the patient populations also lead to conflicting results across studies and warrant further investigation.
Here, we took advantage from a recently developed innovative SPECT system for imaging in laboratory rodents39 to investigate whether the hyperactivity displayed by Fmr1-Δexon 8 rats was accompanied by changes in striatal DAT availability. By performing scintigraphic SPECT analyses in vivo using 123I-FP-CIT as radiolabel, we found comparable DAT availability in the striatum of Fmr1-Δexon 8 rats and WT controls, suggesting that no changes in striatal DAT expression occurred in Fmr1-Δexon 8 rats. Based on these results, it is tempting to speculate that the altered locomotor activity (as expressed by the increased number of crossings in the open field and the increased total entries in the elevated plus maze) we consistently observed in Fmr1-Δexon 8 rats along development might not be attributable to an alteration of striatal DAT availability. This evidence is also corroborated by immunohistochemistry and immunofluorescence analyses, since our results showed no difference in DAT expression between WT and Fmr1-Δexon 8 rats in the dorsal striatum. To evaluate for a possible different contribution of the two distinct districts of the striatum (i.e., dorsal and ventral), we performed Western blot analysis of DAT protein levels: notably, no significant differences were found between genotypes, indicating that DAT protein levels were preserved in both dorsal and ventral striatum of Fmr1-Δexon 8 rats. Despite these consistent results, we cannot exclude that pharmacological manipulation of the dopaminergic system (e.g., administration of D1 and D2 agonist/antagonist, cocaine, or blockers of other transporters) could have an impact on the altered locomotor activity shown by Fmr1-Δexon 8 rats, as previously reported in Fmr1 KO mice83,84,85,86 and DAT-KO mice and rats24,87,88. Moreover, investigating possible DA-independent mechanisms that may drive hyperactivity in Fmr1-Δexon 8 rats also remains an intriguing point that deserves further investigation. For instance, the pharmacological targeting of serotonin89,90, GABA91,92, BDNF93,94, acetylcholine95 pathways (to mention a few) has been demonstrated to ameliorate abnormal locomotor behaviors in Fmr1-KO mice.
Despite dopaminergic aberrations have been extensively documented in Fmr1-KO mice63,96,97,98,99, preclinical data on the striatal DAT activity remain controversial, with some studies reporting a decreased striatal DAT expression97, whereas others (including the present results) showing no change in DAT function99. Based on these (apparent) contradictory findings, we should consider the hypothesis that specific changes in DA signaling may differentially contribute to ASD pathophysiology and consequently may (not) account for the full spectrum of ASD-related behavioral manifestations73. For instance, it has been shown that activation of D2 expressing neurons in the ventral striatum reduced running and locomotion in mice, while D2 expressing neuron inhibition had opposite effects100; moreover, FMRP seems to be involved in D1 -mediated neuroplasticity in the prefrontal cortex101,102,103. Besides, DA receptors are differentially integrated in cortical circuit components subserving distinct aspects of cognitive control, including relaying motor commands104. This will undoubtedly contribute to clarify the cellular (D1 vs. D2) and regional (dorsal vs. ventral striatum, prefrontal cortex, cerebellum) specificity of DA pathways in mediating motor dysfunctions in Fmr1-Δexon 8 rats.
As referring to the SPECT methodology, it is important to clarify that FP-CIT is not a substrate for the transporter, hence imaging analysis only provides DAT expression. Accordingly, it is possible that alterations in the dopaminergic system contribute to the observed hyperactivity phenotype through one of the following mechanisms: (i) altered trafficking or catalytic activity of DAT; (ii) altered synthesis, packaging, or release of DA; (iii) altered sensitivity of DA receptors105. These hypotheses warrant further investigation in a follow-up of this study.
Overall, our results showed that Fmr1-Δexon 8 rats displayed hyperactivity in the open field and in the elevated plus maze tests, in the absence of repetitive behaviors in the hole board test, with no changes in striatal DAT availability as assessed by in vivo SPECT imaging and Western blot experiments. This study supports a preservation of striatal DAT availability following FMR1 deletion in rats and confirms that in vivo SPECT imaging paralleled by behavioral observation represents a useful tool to non-invasively investigate variations in neurotransmitter activity in neurodevelopmental disorders. Since sex-dependent differences in preclinical models of ASD have been documented106, the inclusion of both male and female animals should be considered in future studies.

