Sigma‑1 receptor overexpression promotes proliferation and ameliorates cell apoptosis in β‑cells
By Mengting Ke, Fengping Lin, Huawei Wang, Guangzhen He, Jieyuan Feng, Linyang Song, Yancheng Xu, and Jie Liu
Excerpt from the article published in Molecular Medicine Reports 25, no. 5 (2022): 170. https://doi.org/10.3892/mmr.2022.12686
Editor’s Highlights
- Sigma-1 receptor (Sig-1R) is a class of receptors that serve unique physiological functions due to the lack of homology with other mammalian proteins.
- Sig-1R agonists have a protective effect on Alzheimer’s disease (AD), Parkinson’s disease (PD), heart disease, retinal dysfunction, perinatal and traumatic brain injuries, depression, and psychostimulant addiction.
- The Mitochondria and the endoplasmic reticulum (ER) are important organelles in eukaryotic cells. The Mitochondria‑associated ER membrane (MAM) is the site of physical coupling between the mitochondrial outer membrane and ER, where Sig-1R has been reported to be a chaperone protein.
- MAM enables the direct transport of ER calcium to the mitochondria through the IP3R/GRP75/VDAC1 complexes.
- Sig-1R has been shown to control calcium transport by regulating the formation of these complexes.
- Sig-1R overexpression ameliorated ER stress and mitochondrial dysfunction in pancreatic MIN6 beta-cells by preserving calcium transport between the mitochondria and ER.
- Sig-1R overexpression may exert protective effects on β-cells under lipotoxic conditions.
Abstract
Sigma‑1 receptor (Sig‑1R) is a class of orphan receptors, the potential role of which in pancreatic islet cells remains poorly understood. The present study aimed to investigate the role of Sig‑1R in islet β‑cell proliferation and examine the effects of Sig‑1R on islet β‑cell injury under lipotoxic conditions. Sig‑1R‑overexpressing MIN6 cells were generated by lentiviral vector transfection. The effect of Sig‑1R overexpression on cell proliferation detected by EdU staining, cell cycle progression by propidium iodide (PI), apoptosis by Annexin V‑APC/PI, mitochondrial membrane potential by Mitolite Red and cytoplasmic Ca2+ levelsby Fura‑2/AM in islet β‑cells were measured by flow cytometry. Western blot analysis was used to measure protein expression levels of endoplasmic reticulum (ER) stress markers glucose‑regulated protein 78 and C/EBP homologous protein, mitochondrial apoptotic proteins Bcl‑2‑associated X and Bcl‑2 and cytochrome c. In addition, ATP levels and insulin secretion were separately measured using ATP Assay and mouse insulin ELISA. Mitochondria‑associated ER membrane (MAM) structures in MIN6 cells were then detected using transmission electron microscopy. Protein disulfide isomerase expression and possible colocalization between inositol 1,4,5‑trisphosphate receptor and voltage‑dependent anion channel 1 were examined using immunofluorescence. Sig‑1R overexpression was found to promote β‑cell proliferation by accelerating cell cycle progression. Furthermore, Sig‑1R overexpression ameliorated the apoptosis rate whilst impairing insulin secretion induced by palmitic acid by relieving ER stress and mitochondrial dysfunction in MIN6 cells. Sig‑1R overexpression also promoted Ca2+transport between mitochondria and ER by increasing the quantity of ER adjacent to mitochondria in the 50‑nm range. It was concluded that Sig‑1R overexpression conferred protective effects on β‑cells against lipotoxicity as a result of the promotion of cell proliferation and inhibition of ER stress and oxidative stress, by regulating the structure of MAM.
Introduction
The global prevalence of diabetes continues to increase every year since 1980 (1). In 2019, ~463 million individuals were diagnosed with diabetes, which accounted for 9.3% of the global adult population (2). Type 2 diabetes mellitus (T2DM) is the most common type of diabetes, where β-cell apoptosis is one of the main causes of T2DM (3). Therefore, further studies on the mechanism underlying β-cell apoptosis are essential for developing treatment strategies for T2DM.
Sigma-1 receptor (Sig-1R) is a class of orphan receptors that has been reported to serve unique physiological functions due to the lack of homology with other mammalian proteins (4,5). Sig-1R agonists has a protective effect on Alzheimer’s disease (AD), Parkinson’s disease (PD), heart disease, retinal dysfunction, perinatal and traumatic brain injuries, depression and psychostimulant addiction (6). Sig-1R agonists are effective neuroprotective agents (7) and have been applied for treating various neurodegenerative diseases, such as AD, PD and amyotrophic lateral sclerosis (8). In human lens cells, Sig-1R antagonists can inhibit cell proliferation (9), suggesting that Sig-1R can exert regulatory effects on cell proliferation. In addition, Sig-1R receptor agonists have been found to increase the viability of human retinal pigment epithelial cells following oxidative damage (10). Treatment of mice with Sig-1R agonists following transient middle cerebral artery occlusion was reported to improve the extent of cerebral ischemic injury by relieving endoplasmic reticulum (ER) stress (11). Sig-1R receptor agonists can also reduce C/EBP homologous protein (CHOP) expression in HEK cells to attenuate the ER stress-mediated apoptotic pathway (12). Finding from these previous studies implicated regulatory effects of Sig-1R on ER stress. Since ER stress has been demonstrated to be an important mechanism of islet cell apoptosis in patients with T1DM and T2DM (13,14), it is speculated that Sig-1R activation can also mediate protective effects on islet cells. However, the role of Sig-1R in pancreatic islet cells remains poorly understood. Therefore, the present study investigated the potential link between Sig-1R and islet cell function, by investigating the effects of Sig-1R overexpression on β-cellphysiology.
…
Results
Generation of Sig-1R-overexpressing cells
Lentiviral vectors were used to transfect MIN6 cells to create the Sig-1R-overexpressing Lv-Sig-1R cells. RT-qPCR revealed a significant increase in Sig-1R mRNA expression in Lv-Sig-1R cells compared with that in the Lv-control cells (P<0.05; Fig. 1A). Subsequent western blot analysis also showed a significant increase in Sig-1R protein expression in Lv-Sig-1R cells compared with that in Lv-control cells (P<0.05; Fig. 1B).
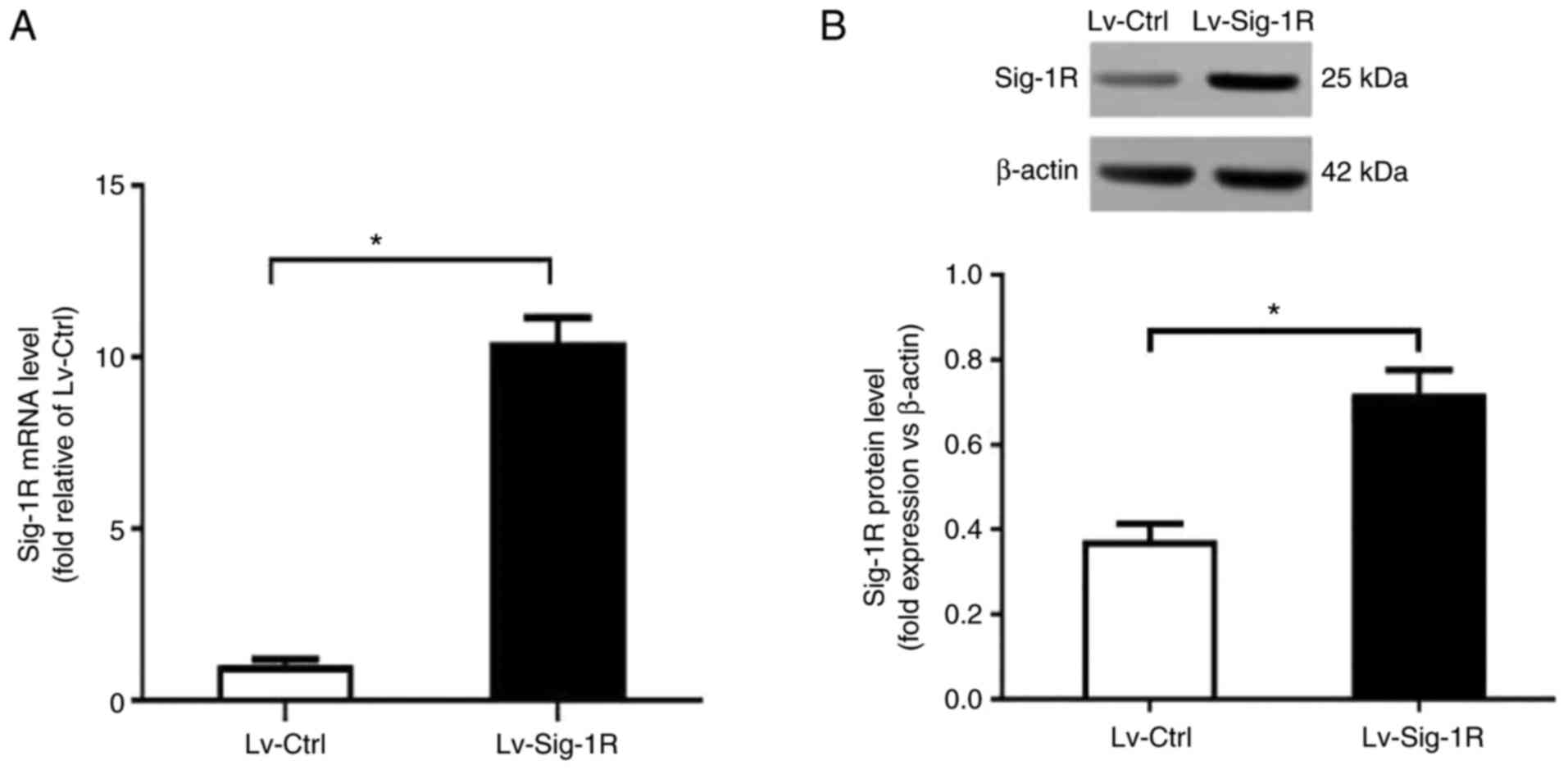
Establishment of Sig-1R-overexpressing MIN6 cells. (A) Sig-1R mRNA and (B) protein expression in Lv-Sig-1R and Lv-Ctrl cells were measured. The results are means ± SD fromthree experiments. *P<0.05. Sig-1R, Sigma-1 receptor; Lv, lentiviral; Ctrl, control.
Sig-1R overexpression promotes proliferation and cell cycle progression
EdU incorporation assay showed that Lv-Sig-1R cells had a significantly increased percentage of EdU-positive cells compared with that in the Lv-Ctrl cells (P<0.05; Fig. 2A and B), suggesting that Sig-1R overexpression promoted MIN6 cell proliferation. Cell cycle analysis demonstrated that the percentage of cells in G1 phase was significantly decreased whereas that in S phase was significantly increased, in Lv-Sig-1R cells compared with that in Lv-Ctrl cells (P<0.05; Fig. 2C and D). These results suggest that Sig-1R overexpression promoted β-cell proliferation resulting from the potentiation of cell cycle progression.
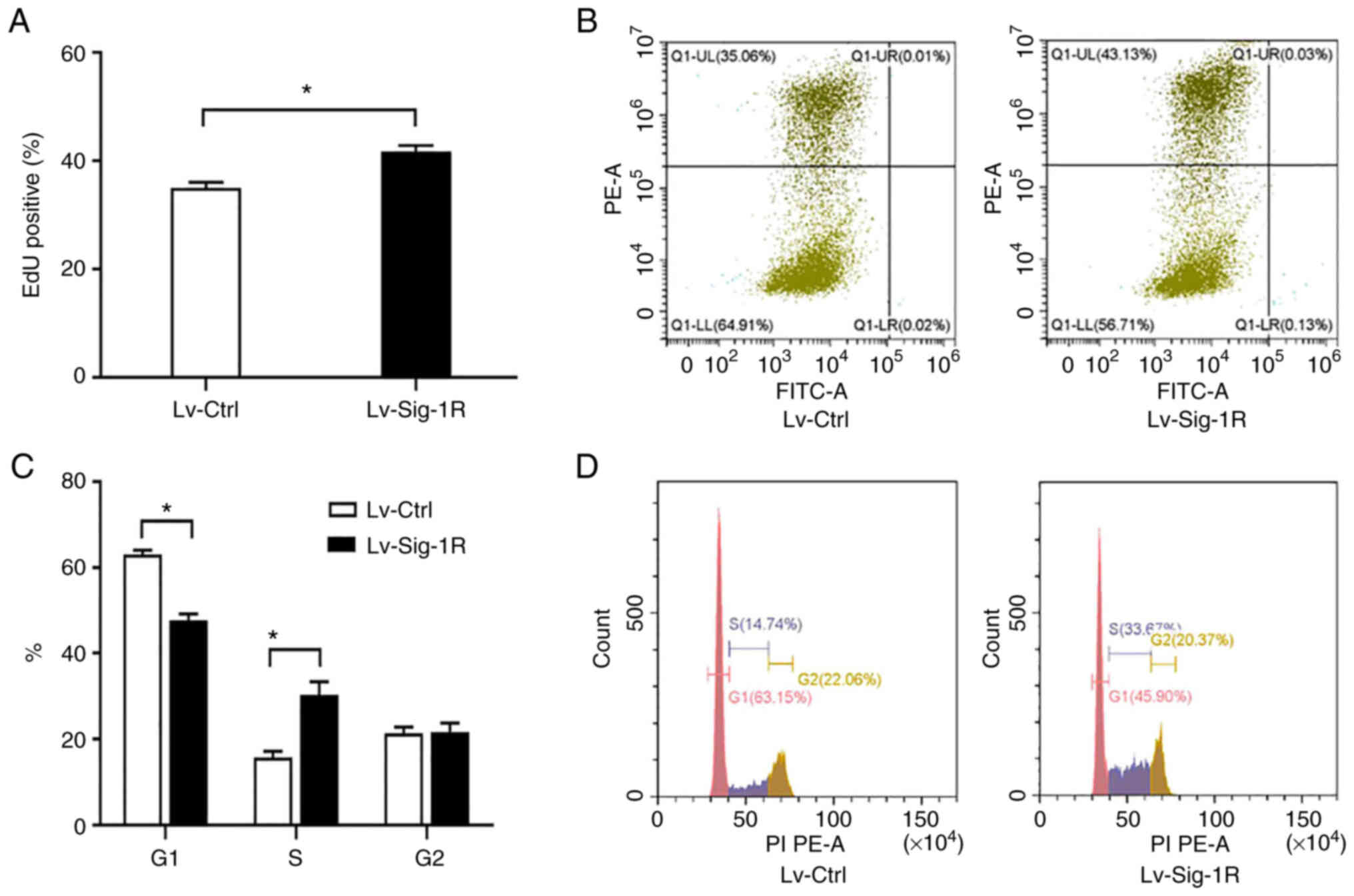
Effect of Sig-1R overexpression on MIN6 cell proliferation and cell cycle progression. (A) Quantification of the percentage of EdU-positive cells in Lv-Sig-1R cells and Lv-Ctrl cells. (B) Representative flow cytometry diagrams showing the percentage of EdU-positive cells (Q1-UL represents the EdU-positive cellsand Q1-LL represents the EdU-negative cells. GFP was detected by FITC and EdU was detected by PE). (C) Quantification of the percentage of cells in the three different phases of cell cycle in Lv-Sig-1R cells and Lv-Ctrl cells. (D) Representative flow cytometry histograms showing the percentage of cells in the different cell cycle phases. The results represent the means ± SD fromthree experiments. *P<0.05. Sig-1R, Sigma-1 receptor; Lv, lentiviral; Ctrl, control.
Sig-1R overexpression prevents apoptosis and impairs PA-induced insulin secretion in MIN6 cells
Cell apoptosis assay revealed similar cell apoptosis rates under basal conditions in both Lv-Ctrl and Lv-Sig-1R cells. However, the apoptosis rate significantly increased after PA was added in both groups of these cells (P<0.05; Fig. 3). In particular, the cell apoptosis rate increased by a larger extent in Lv-Ctrl cells compared with that in Lv-Sig-1R cells after exposure to PA (P<0.05; Fig. 3).
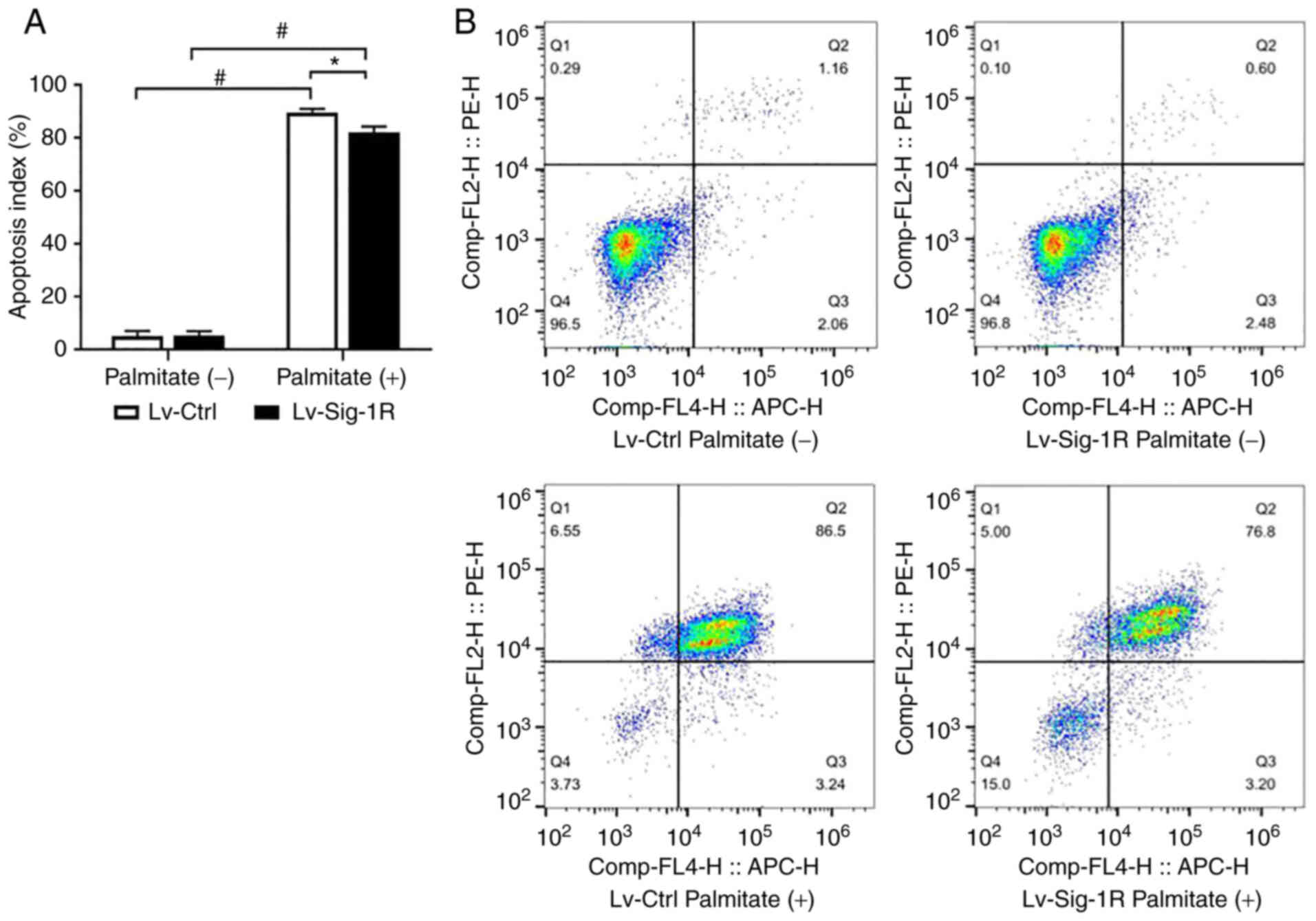
Effect of Sig-1R overexpression on palmitate-induced MIN6 cell apoptosis. (A) Quantification of the cell apoptosis rate in Lv-Sig-1R cells and Lv-Ctrl cells. (B) Representative flow cytometry diagrams showing the cell apoptosis rate. Q2 represents late apoptotic cells and Q3 represents early apoptotic cells. The results represent the means ± SD from three experiments. *P<0.05 and #P<0.05. Sig-1R, Sigma-1 receptor; Lv, lentiviral; Ctrl, control.
Glucose-stimulated insulin secretion assay showed that insulin secretion was significantly decreased in Lv-Ctrl and Lv-Sig-1R cells after exposure to PA compared with that in their corresponding control that were not treated with PA (P<0.05; Fig. 4A). However, significantly increased insulin secretion was observed in Sig-1R-overexpressing MIN6 cells compared with that in Lv-Ctrl cells regardless of whether they were exposed to palmitate (P<0.05; Fig. 4A). Therefore, it was concluded that Sig-1R overexpression ameliorated PA-induced impaired insulin secretion and cell apoptosis.
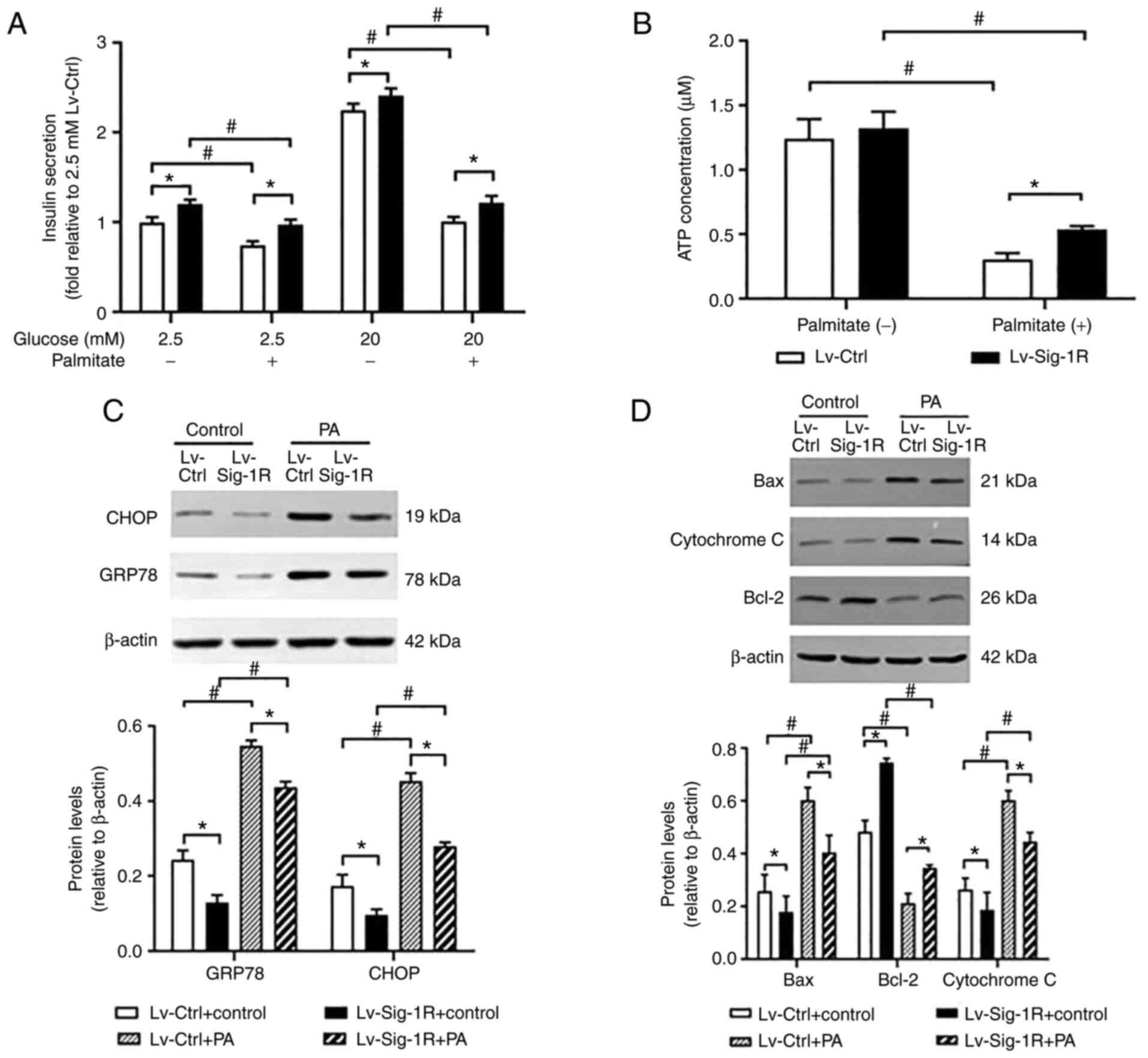
Effect of Sig-1R overexpression on PA- and glucose-induced insulin secretion, ATP production and ER stress in MIN6 cells. (A) Levels of insulin secretionby Lv-Sig-1R cells and Lv-Ctrl cells. (B) Levels of ATP production by Lv-Sig-1R cells and Lv-Ctrl cells. (C) Levels of GRP78 and CHOP protein expression in Lv-Sig-1R cells and Lv-Ctrl cells. (D) Expression of mitochondria-associated apoptotic proteins Bax, Bcl-2 and Cytochrome c in Lv-Sig-1R cells and Lv-Ctrl cells. The results are means ± SD for three observations; *P<0.05 and #P<0.05. ER, endoplasmic reticulum; GRP78, glucose-regulated protein 78; CHOP, C/EBP homologous protein; Sig-1R, Sigma-1 receptor; Lv, lentiviral; Ctrl, control; PA, palmitic acid.
Sig-1R overexpression relieves ER stress and mitochondrial dysfunction induced by PA in MIN6 cells
GRP78 and CHOP are typical markers of ER stress (18). The results in the present study showed that the protein expression of GRP78 and CHOP in Lv-Ctrl and Lv-Sig-1R cells was both significantly increased on exposure to PA compared with that in their corresponding cells not treated with PA (P<0.05; Fig. 4C). Similar to the insulin secretion data, after treatment with cells with treated with PA, protein expression of GRP78 and CHOP was significantly decreased in Sig-1R-overexpressing MIN6 cells compared with that in Lv-Ctrl cells (P<0.05; Fig. 4C). PDI promotes the correction of disulfide bonds between proteins. During the early stages of ER stress, PDI is typically activated to maintain ER stability by reducing the aggregation of misfolded and unfolded proteins within ER (19). Immunofluorescence results showed that PDI expression was markedly decreased in Sig-1R-overexpressing MIN6 cells compared with that in Lv-Ctrl cells following the exposure of both cells to PA (Fig. 5). These results suggest that Sig-1R overexpression can relieve ER stress induced by PA in MIN6 cells.
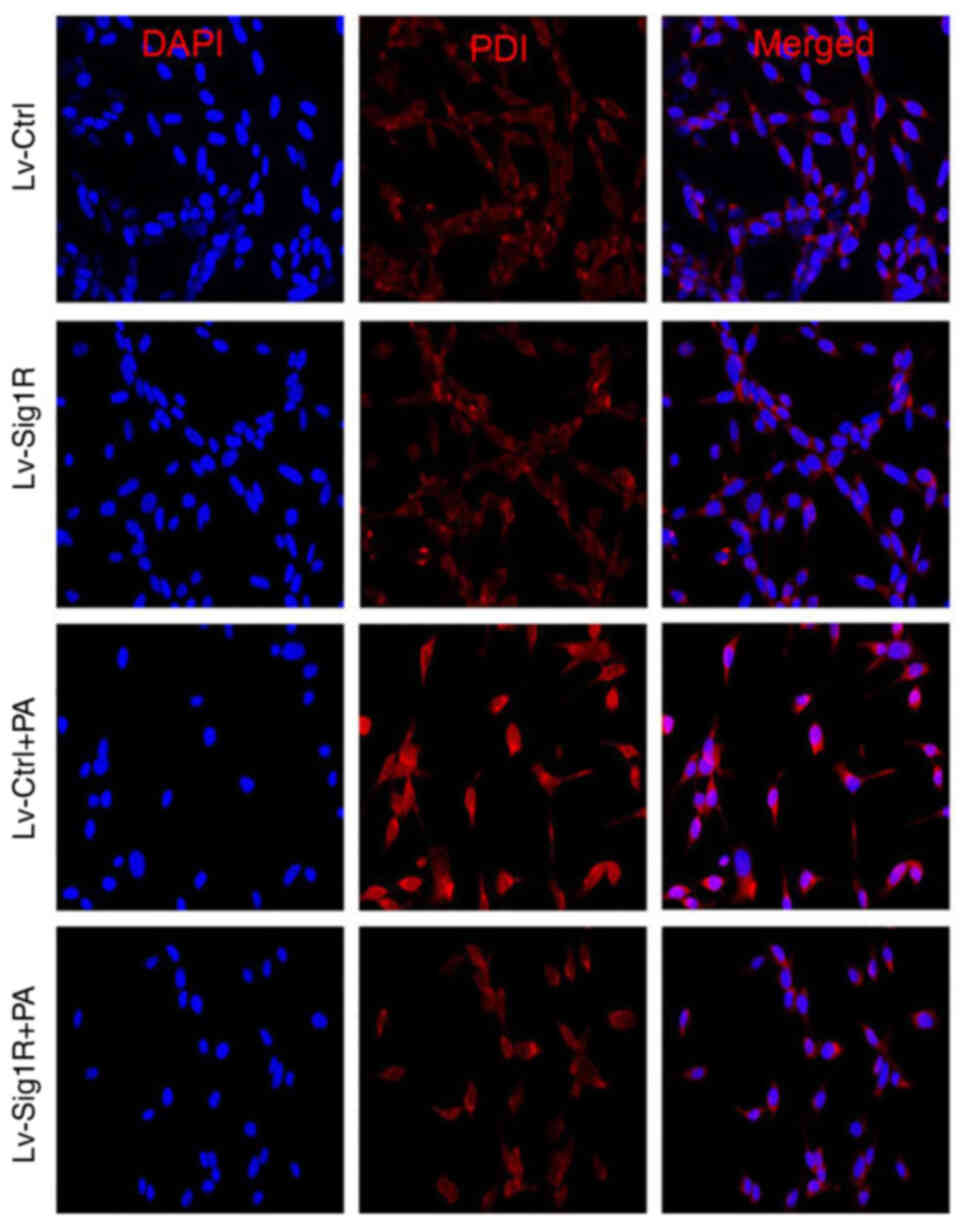
Effect of Sig-1R overexpression on PDI expression in MIN6 cells. Representative images of PDI expression by immunofluorescence in Lv-Sig-1R and Lv-Ctrl cells. Magnification, ×400. Sig-1R, Sigma-1 receptor; Lv, lentiviral; Ctrl, control; PA, palmitic acid; PDI, protein disulfide isomerase.
During oxidative stress, Bax and cytochrome c are released from mitochondria into cytoplasm and activate caspase 9 to induce apoptosis, suggesting that Bax and cytochrome c are mitochondria-associated apoptosis proteins (20). Downstream, ATP and MMP can be used to reflect mitochondrial function. After PA intervention, Bax and cytochrome c protein levels were significantly increased, whilst ATP, MMP and Bcl-2 expression were significantly decreased. Bax and cytochrome c expression were significantly increased in the Sig-1R overexpression group (Fig. 4D), whereas ATP (Fig. 4B), MMP (Fig. 6) and Bcl-2 expression levels (Fig. 4D) were significantly decreased (P<0.05) compared with that in Lv-Ctrl cells following the exposure of both cells to PA. Therefore, these results suggest that Sig-1R overexpression alleviated PA-induced mitochondrial dysfunction.
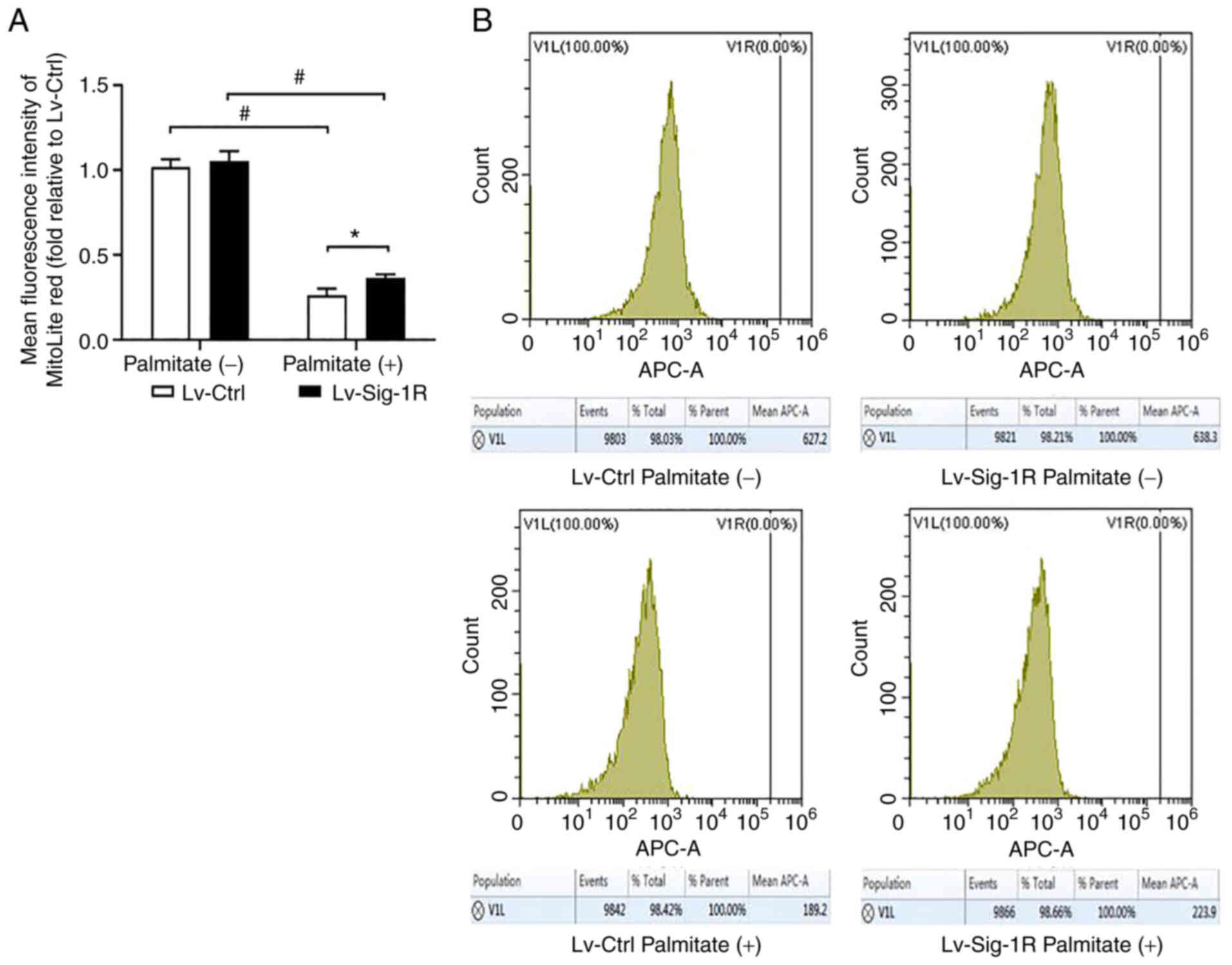
Effect of Sig-1R overexpression on the mitochondrial membrane potential in MIN6 cells. (A) Quantification of mitochondrial membrane potential levels in Lv-Sig-1R cells and Lv-Ctrl cells. (B) Representative flow cytometry histogram showing the average fluorescence intensity of Mitolite Red, which is the fluorescent probe used to detect the mitochondrial membrane potential level. The results are shown as the means ± SD from three experiments. *P<0.05 and #P<0.05. Sig-1R, Sigma-1 receptor; Lv, lentiviral; Ctrl, control.
Sig-1R overexpression alters the structure of mitochondria-associated membranes (MAM)
Mitochondrial and ER are important organelles within nucleated eukaryotic cells that are key to intracellular cell physiology. They are arranged in parallel, where various points of physical coupling exists between the outer mitochondrial membrane and the ER, which are called the MAM (21). The MAM contains a plethora of functional proteins that regulate the transport of metabolites and signaling molecules, such as Sig-1R (22). Therefore, it was hypothesized that Sig-1R overexpression may alter the structure of MAM. The present study next examined the structure of MAM using TEM. An increase in the quantity of ER adjacent to mitochondria was observed in the 50-nm range according to TEM analysis in Lv-Sig-1R cells compared with that in Lv-control cells (Fig. 7). Subsequently, immunofluorescence was used to detect the effect of Sig-1R overexpression on the expression and localization of key MAM proteins IP3R and VDACI. The expression level of VDAC1was markedly increased in Sig-1R-overexpressing MIN6 cells compared with that in Lv-control cells without PA. Although the expression level of the two proteins decreased in the PA group, VDAC1 expression remained to be higher in Sig-1R-overexpressing MIN6 cells. On the other hand, Sig-1R overexpression had no significant effect on IP3R protein expression with or without PA compared with that in Lv-control cells (Fig. 8). These results suggested that Sig-1R overexpression had an effect on the structure of MAM by increasing the expression of VDAC1.
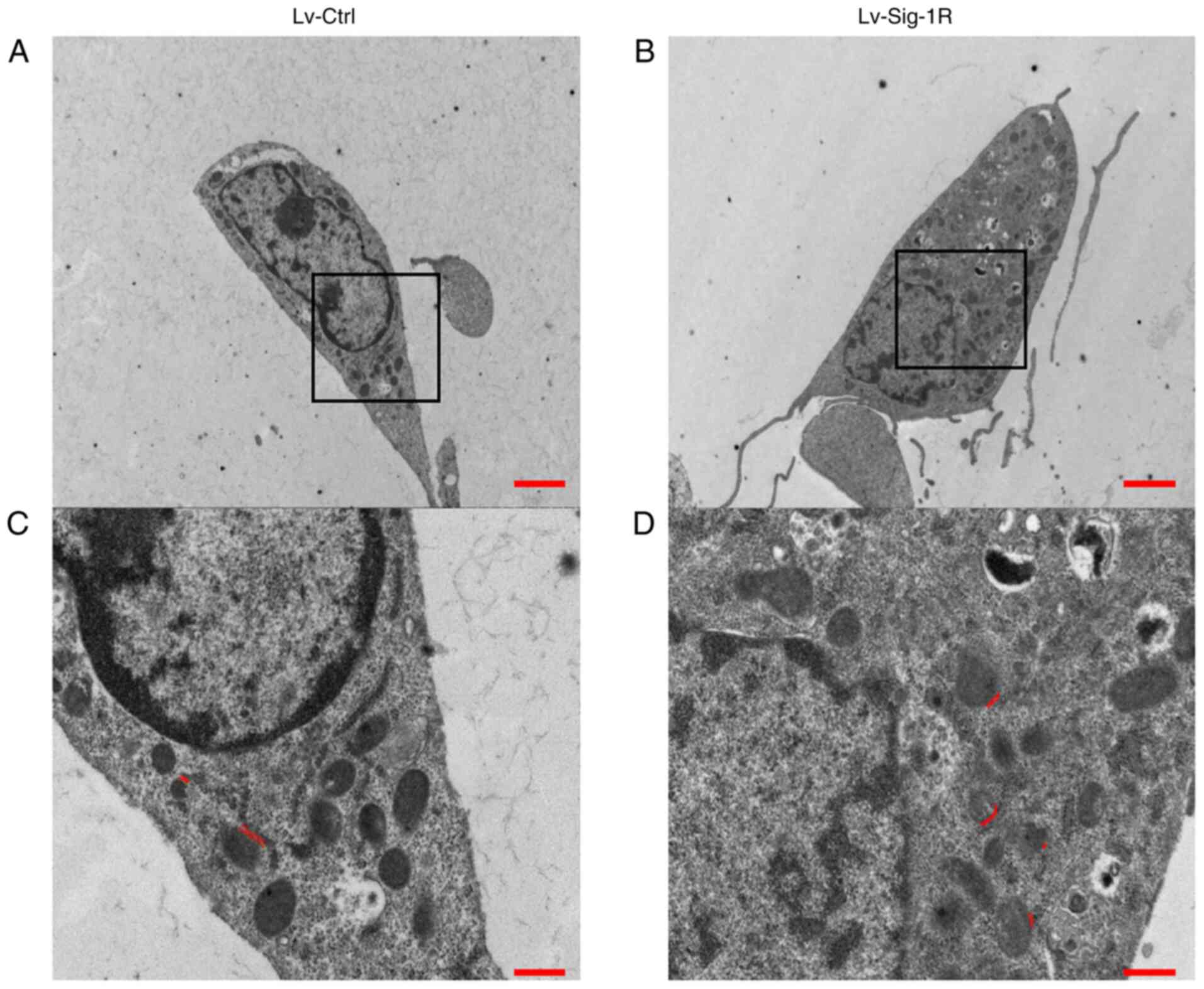
Effect of Sig-1R overexpression on the mitrochondria-endoplasmic reticulum junction morphology. Representative transmission electron microscopy images of the whole cell for (A) Lv-Ctrl cells and (B) Lv-Sig-1R cells. Scale bars, 20 µm. Representative images of the amplified sections for (C) Lv-Ctrl cells and (D) Lv-Sig-1R cells of the corresponding black box content in (A) and (B), respectively. Scale bars, 5 µm. The double red line shows the location of the mitochondria-endoplasmic reticulum coupling structure in the range of 50 nm. Sig-1R, Sigma-1 receptor; Lv, lentiviral; Ctrl, control.
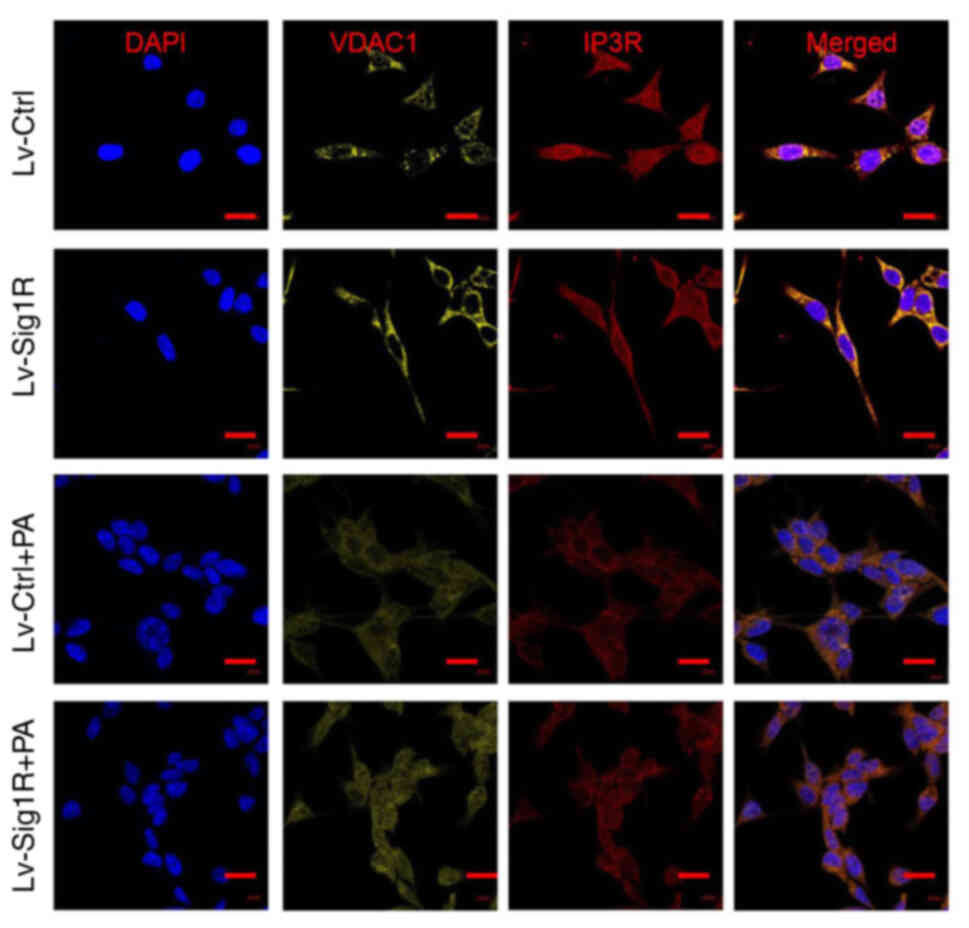
Effect of Sig-1R overexpression on the mitochondria-associated ER membrane junction morphology. Representative immunofluorescence images of IP3R-VDACI on the mitochondria-associated ER membrane in Lv-Sig-1R cells with Lv-Ctrl cells, which was imaged using confocal microscopy. Scale bars, 10 µm. PA, palmitic acid; IP3R, inositol 1,4,5-triphosphate receptor; VDAC, voltage-dependent anion channel 1; Sig-1R, Sigma-1 receptor; Lv, lentiviral; Ctrl, control.
Sig-1R overexpression increases cytoplasmic calcium level
Fura 2/AM is a class of cytoplasmic calcium probe that can be used to reflect cytoplasmic calcium levels. The present study revealed that the cytoplasmic calcium level was significantly increased in the PA group compared with that in the group not treated with PA in both Lv-control and Lv-Sig-1R cells (Fig. 9). In addition, the cytoplasmic calcium level was found to be significantly lower in Sig-1R-overexpressing cells compared with that in control cells in the absence of PA (Fig. 9).
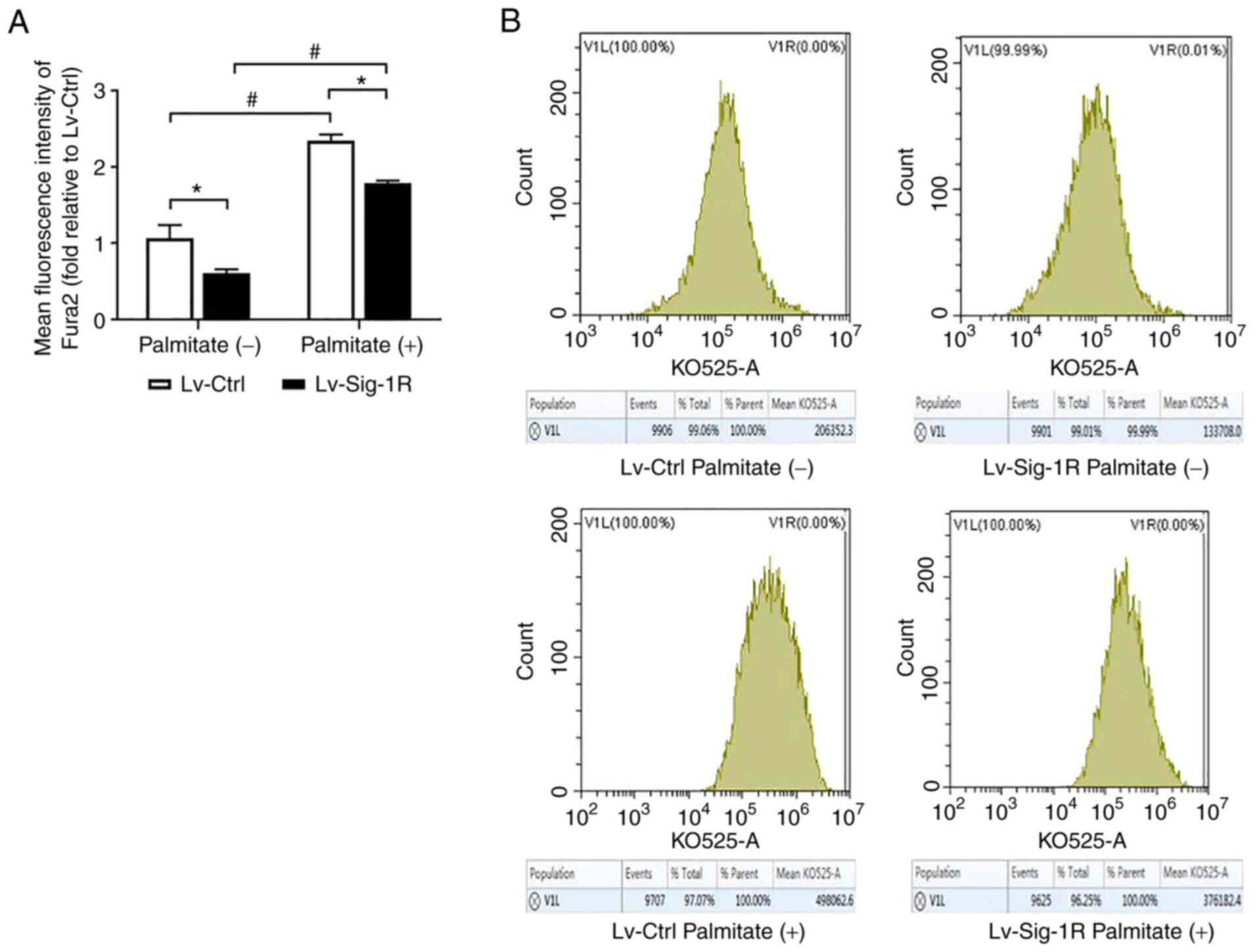
Effect of Sig-1R overexpression on the intracellular calcium levels of MIN6 cells. (A) Quantification of cytoplasmic calcium levels in Lv-Sig-1R cells and Lv-Ctrl cells. (B) Representative flow cytometry histograms showing the average fluorescence intensity of Fura 2, which is a fluorescent probe used to detect cytoplasmic calcium levels. The results are representative of the mean ± SD of three experimental repeats. *P<0.05 and #P<0.05. Sig-1R, Sigma-1 receptor; Lv, lentiviral; Ctrl, control.
Discussion
Sig-1R is a class of receptors that have unique pharmacological effects and chaperone activity (23). In human lens cells, Sig-1R receptor antagonists have been shown to inhibit cell proliferation (9). In the present study, Sig-1R overexpression was found to increase the proliferation rate of MIN6 cells. Cell cycle progression serves a key role in regulating cell proliferation, where the transition from G0/G1 to the S phase is a key step in this process (24). To explore the mechanism further, the cell cycle progression analysis of MIN6 cells in the present study revealed that Sig-1R overexpression this process, specifically from the G1 to the S phase. Therefore, it was hypothesized that Sig-1R can promote cell proliferation by positively regulating the cell cycle.
Numerous studies have shown that exposure to PA can decrease insulin secretion and induce apoptosis in β-cells (25,26). Apoptosis and Glucose-stimulated insulin secretion assays in the present study also showed that exposure to PA increased apoptosis whilst decreasing insulin secretion, which was consistent with these previous findings (25,26). In addition, Sig-1R overexpression was found to ameliorate apoptosis and restored the insulin secretion previously impaired by PA in MIN6 cells. Sig-1R agonists have been previously shown to alleviate cerebral ischemia-reperfusion injury by reversing neuronal apoptosis and improving neurological function (27). Amyotrophic lateral sclerosis is a progressive neurological disorder (28). In this disease, brain neuronal apoptosis was found to be inhibited after prolonged treatment with Sig-1R agonists (28). Altogether, these findings suggest that Sig-1R mediates protective effects against cell damage, whereby increasing Sig-1R activity can alleviate cell damage.
Therefore, the underlying mechanism was explored. Oxidative and ER stress are associated with islet β-cell dysfunction and participate in the development of T2DM (29). Results in the present study showed that Sig-1R overexpression relieved PA-induced ER stress in MIN6 cells by decreasing the protein expression of the ER chaperone GRP78 and the ER pro-apoptotic molecule CHOP. Previous studies also reported that Sig-1R serves important roles in ER stress (11,30). Sig-1R upregulation was found to alleviate neuronal damage caused by ER stress (11), whereas the activation of Sig1R effectively inhibited the expression of GRP78 and CHOP to alleviate apoptosis in mouse hippocampal cells (30). PDI promotes the formation of correct disulfide bonds between and/or within proteins (31). During the early stages of ER stress, PDI is activated to maintain stability by reducing the aggregation of misfolded and unfolded proteins within ER (19). The present showed that PDI expression was decreased in the Sig-1R-overexpressing MIN6 cells compared with that in Lv-Ctrl cells after exposure to PA. Taken together, these findings suggested that Sig-1R overexpression can relieve MIN6 cell apoptosis under lipotoxic conditions through ameliorating ER stress. Furthermore, the present study also concluded that Sig-1R overexpression can alleviate PA-induced mitochondrial dysfunction in MIN6 cells. Previous studies showed that Sig1R agonists relieve oxidative stress (32) and promote ATP production (33). Tagashira et al (34) found that ligands that can activate Sig-1R can protect cardiomyocytes by increasing IP3R-mediated mitochondrial ATP production. Therefore, Sig-1R overexpression was concluded to ameliorate apoptosis and restore insulin secretion under lipotoxic conditions by relieving ER stress and mitochondrial dysfunction in MIN6 cells.
Mitochondrial and ER are important organelles in eukaryotic cells. Although the two organelles are normally in close proximity to each other, their membranes do not fuse (35). Therefore, both can retain their own unique structure and function (35). MAM is the site of physical coupling between the mitochondrial outer membrane and ER, where Sig-1R has been reported to be a chaperone protein (22). Sig-1R knockdown can lead to AD, the mechanism of which may be associated with the loss of MAM integrity (36). Therefore, it was speculated in the present study that Sig-1R can regulate the MAM structure in islet cells, thereby attenuating islet apoptosis by regulating MAM structure by regulating ER stress and mitochondrial function. An increase in the number of ER and mitochondria contacts was observed in the 50-nm range by TEM analysis in Lv-Sig-1R cells, suggesting that Sig-1R overexpression serves an important role in promoting the formation of MAM.
IP3R is one of the calcium release channels in the ER (37). When IP3R interacts with VDAC1 on the outer mitochondrial membrane using the molecular chaperone glucose regulatory protein 75 (GRP75) bridge, calcium ions are released from the ER directly through IP3R without the combination between IP3 and IP3R (38). The IP3R/GRP75/VDAC1 complex is a multi-protein structure that is associated with the coupling of the mitochondrial cytoplasmic network, where GRP75 knockdown can prevent MAM formation and reduce mitochondrial calcium uptake (39). Immunofluorescence results from the present study showed that Sig-1R overexpression mainly increased the expression levels of VDAC1. This suggests that Sig-1R mediated a regulatory effect on the structure of MAM by increasing the expression of VDAC1.
MAM enables the direct transport of ER calcium to the mitochondria through the IP3R/GRP75/VDAC1 complexes (40). Sig-1R has been shown to control calcium transport by regulating the formation of these complexes (41). The present study showed that the cytoplasmic calcium levels were decreased in Sig-1R-overexpressing MIN6 cells compared with that in Lv-Ctrl cells. This may have been because Sig-1R overexpression increased direct calcium transport from the ER to the mitochondria by increasing the number of mitochondria-ER coupling sites, which in turn reduced calcium leakage into the cytosol. Therefore, it was also speculated that Sig-1R overexpression ameliorated ER stress and mitochondrial dysfunction in MIN6 cells by preserving calcium transport between the mitochondria and ER.
To conclude, the present study revealed that Sig-1R overexpression may exert protective effects on β-cells under lipotoxic condition. This could be because Sig-1R overexpression not only promoted cell proliferation but also relieved ER stress and mitochondrial dysfunction. However, several limitations remain. Only one cell line was used. It should be verified further in primary β-cells or in β-cells in vivo. The specific mechanism of how Sig-1R regulates cell apoptosis require further investigation, such that cleaved caspase 3 expression levels should be tested.

