Sigma-1 Receptor Promotes Mitochondrial Bioenergetics by Orchestrating ER Ca2+ Leak during Early ER Stress
By Koshenov, Z.; Oflaz, F.E.; Hirtl, M.; Pilic, J.; Bachkoenig, O.A.; Gottschalk, B.; Madreiter-Sokolowski, C.T.; Rost, R.; Malli, R.; Graier, W.F.
Excerpt from the article published in Metabolites 2021, 11, 422. Published: 26 June 2021, DOI: https://doi.org/10.3390/metabo11070422
Editor’s Highlights
- The endoplasmic reticulum (ER) is a complex, multifunctional organelle of eukaryotic cells and responsible for the trafficking and processing of nearly 30% of all human proteins.
- Any disturbance to these processes can cause ER stress, which initiates an adaptive mechanism called unfolded protein response (UPR) to restore ER functions and homeostasis.
- Mitochondrial Adenosine triphosphate (ATP) production is necessary to meet the high energy demand of the UPR.
- The regulation of mitochondrial bioenergetics during ER stress is essential to combat many pathologies involving ER stress, the UPR, and mitochondria.
- The Sigma-1 Receptor (S1R) plays a crucial role during the early stages of ER stress, whereby it promotes mitochondrial bioenergetics by directed Ca2+ mobilization and protects against mitochondrial reactive oxygen species (ROS) elevation.
- Additionally, S1R is essential for ER homeostasis under unstressed conditions.
- ER stress and S1R deficiency are involved in various neurodegenerative disorders, such as Alzheimer’s disease, and cancer.
Abstract
The endoplasmic reticulum (ER) is a complex, multifunctional organelle of eukaryotic cells and responsible for the trafficking and processing of nearly 30% of all human proteins. Any disturbance to these processes can cause ER stress, which initiates an adaptive mechanism called unfolded protein response (UPR) to restore ER functions and homeostasis. Mitochondrial ATP production is necessary to meet the high energy demand of the UPR, while the molecular mechanisms of ER to mitochondria crosstalk under such stress conditions remain mainly enigmatic. Thus, better understanding the regulation of mitochondrial bioenergetics during ER stress is essential to combat many pathologies involving ER stress, the UPR, and mitochondria. This article investigates the role of Sigma-1 Receptor (S1R), an ER chaperone, has in enhancing mitochondrial bioenergetics during early ER stress using human neuroblastoma cell lines. Our results show that inducing ER stress with tunicamycin, a known ER stressor, greatly enhances mitochondrial bioenergetics in a time- and S1R-dependent manner. This is achieved by enhanced ER Ca2+leak directed towards mitochondria by S1R during the early phase of ER stress. Our data point to the importance of S1R in promoting mitochondrial bioenergetics and maintaining balanced H2O2 metabolism during early ER stress.
1. Introduction
Among a wide array of functions attributed to the endoplasmic reticulum (ER), its protein folding and Ca2+signaling capabilities are perhaps the most studied ones. These functions require meticulous control and regulation, where disturbance of either can result in ER stress with serious pathological outcomes [1]. ER stress represents an inability of ER to properly fold and process proteins in its lumen, resulting in their accumulation and disruption of regular ER functions. There are three main proteins that sense and act upon ER stress, namely, double-stranded RNA-dependent protein kinase (PRK)-like ER kinase (PERK), activating transcription factor 6 (ATF6), and inositol requiring enzyme 1 (IRE1), which form the core of ER unfolded protein response (UPR) [2]. The primary function of UPR is to restore ER homeostasis following ER stress and involves halt of new protein synthesis, while increasing ER chaperone gene expression. These processes are accompanied by the removal of unfolded proteins (ERAD) from the ER lumen [3]. Importantly, since UPR is energetically demanding, the ER requires more ATP, mainly supplied by mitochondria [4]. Hence, it has been reported that ER-mitochondria tethering is increased during the early stages of ER stress to exchange Ca2+ more efficiently and to promote mitochondrial ATP production [5]. Nevertheless, prolonged ER stress and UPR were shown to lead to apoptosis, due to mitochondrial Ca2+ overload [6]. Sigma-1 receptor (S1R), an ER chaperone mainly residing in mitochondria-associated ER membranes (MAMs), was implicated in both Ca2+ signaling and cell survival during ER stress [7]. Under ER stress, S1R was shown to interact with several prominent ER proteins, including inositol 1,4,5-trisphosphate receptor 3 (IP3R3) and IRE1. Association of S1R with IP3R3 was shown to stabilize the latter at the MAMs and increase ER-mitochondrial Ca2+signaling [7], whereas interaction of S1R with IRE1 was shown to sustain prolonged UPR by IRE1 [8]. Numerous reports demonstrated the involvement of ER stress and S1R deficiency in various neurodegenerative diseases [9,10] and cancer [11], emphasizing the importance of elucidating the exact role of S1R in ER stress. In this work, we scrutinize the involvement of S1R in promoting mitochondrial bioenergetics during the early phases of ER stress by imaging mitochondrial and ER activities in single cells with high-resolution fluorescence microscopy. Using SH-SY5Y neuroblastoma cells, we have been able to demonstrate that S1R is indispensable for increased mitochondrial bioenergetics during early ER stress. We could show that this is achieved by an ER Ca2+ leak that is directed towards mitochondria by S1R.
2. Results
2.1. XBP1 Splicing Increases during Early ER Stress and Is Not Affected by Sigma-1 Receptor Knock-Down
To induce ER stress, SH-SY5Y neuroblastoma cells were treated with 0.6 µM tunicamycin for 2 h and 8 h. The X-box binding protein 1 (XBP1) splicing by IRE1, a well-established marker of ER stress [12,13], was not changed by S1R knock-down (S1R KD), while it was increased in cells treated with tunicamycin for 2 h that dramatically increased upon 8 h treatment (Figure 1a–c).
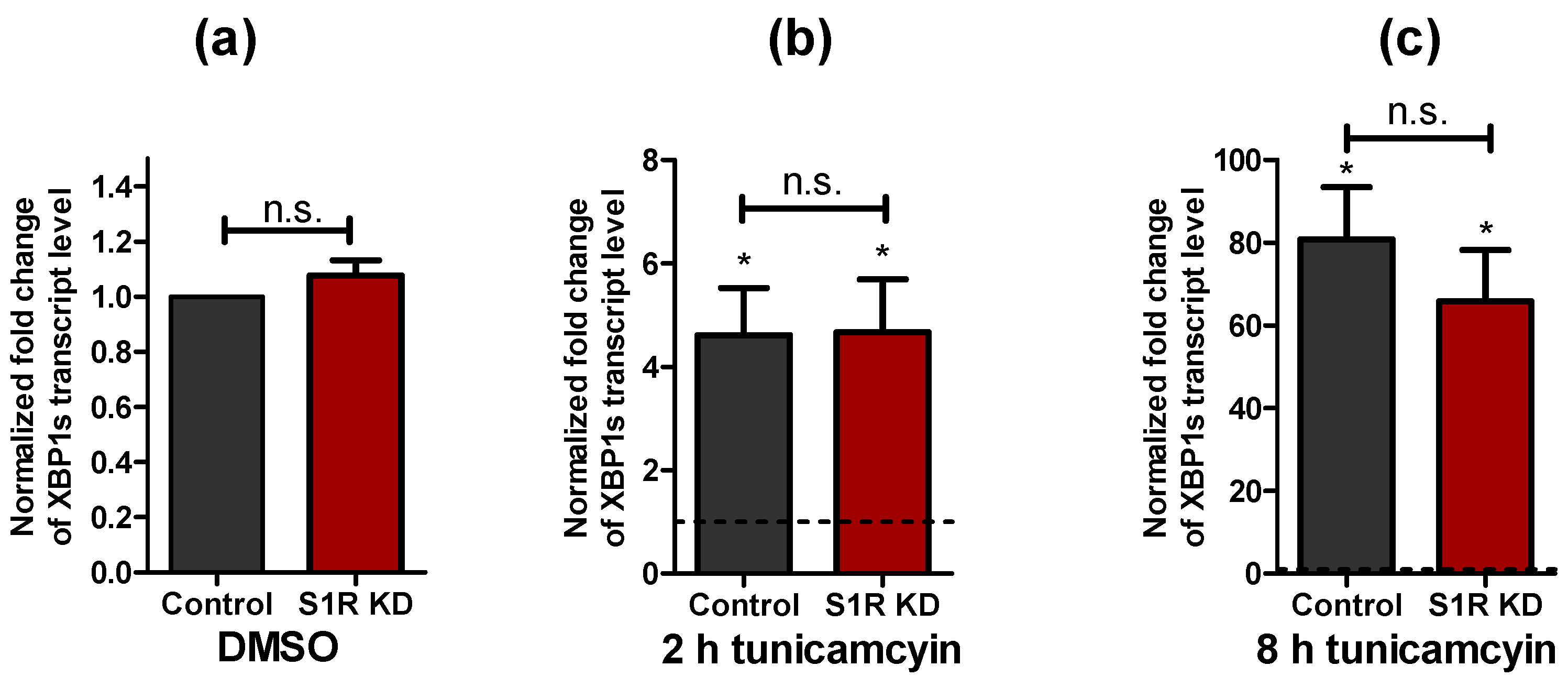
Impact of tunicamycin treatment on XBP1 splicing levels. Bar graphs represent mean ± SEM of spliced XBP1 transcript levels in control and S1R KD SH-SY5Y neuroblastoma cells under control conditions (a) or after tunicamycin treatment for 2 h (b) and 8 h (c). Dashed lines represent the transcript level of spliced XBP1 in the respective DMSO treated cells. Paired t-test, n = 3, * p < 0.05 against corresponding DMSO treated cells; unpaired t-test between control and S1R KD, n = 3, n.s.—not significant.
Since the majority of the experiments in this work were performed using single cells co-transfected with a genetically encoded sensor and siRNA, we quantified the knock-down efficiency in co-transfected GFP-positive sorted cells, which showed much higher knock-down efficiency compared to unsorted cells (Figure S1a,b).
2.2. Sigma-1 Receptor Is Essential for Increased Mitochondrial ATP Level during Early ER Stress
To assess the impact of S1R on mitochondrial bioenergetics during the early phases of ER stress, we measured mitochondrial ATP levels of SH-SY5Y cells using a genetically encoded mitochondria-targeted ATP probe, mtAT1.03 [14] after 2 h and 8 h of tunicamycin treatment. Mitochondrial ATP was maximally increased after 2 h and slightly after 8 h of tunicamycin treatment of control cells (Figure 2a). Basal mitochondrial ATP levels remained unaffected in cells treated with siRNA against S1R (Figure 2a,b). The mitochondrial ATP increases in response to tunicamycin at both time points were, however, abolished by S1R knock-down (Figure 2b), indicating the involvement of S1R in raising mitochondrial ATP during tunicamycin-induced ER stress. To further validate these results, we have used a selective S1R antagonist, BD1047 [15]. Similar to the knock-down of S1R, BD1047 did not affect basal ATP levels within mitochondria, but greatly attenuated the mitochondrial ATP elevations in response to 2 h and 8 h tunicamycin treatment (Figure 2c), thus supporting the need for S1R to increase mitochondrial ATP during early ER stress. Interestingly, neither knock-down nor BD1047 affected mitochondrial ATP levels without tunicamycin treatment (Figure 2d), implying the importance of S1R for mitochondrial ATP production primarily during ER stress.
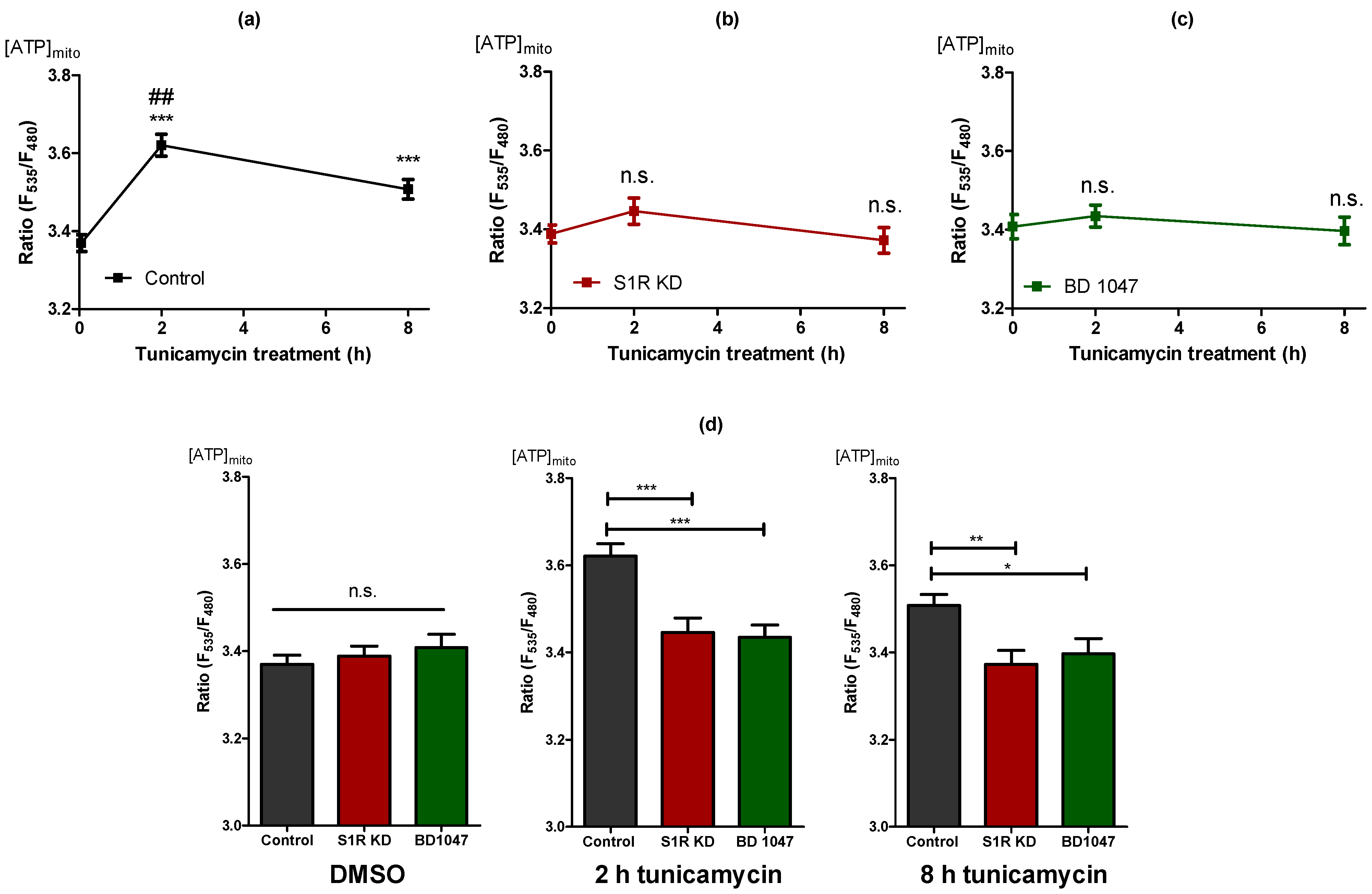
The increase in mitochondrial ATP levels during early ER stress is dependent on S1R. Time course of mitochondrial ATP levels after tunicamycin treatment, presented as mean ± SEM and assessed by the mitochondrial-targeted ATP biosensor AT1.03 in control SH-SY5Y cells (a), in cells with S1R KD (b) or after treatment with BD 1047 (c). (d) Bar graphs represent MEAN ± SEM of mitochondrial ATP levels in control (black), S1R KD (red), and BD1047 treated (green) cells before tunicamycin treatment (left), after 2 h (middle), or 8 h (right) tunicamycin treatment. One-way ANOVA with Tukey’s multiple comparison test, * p < 0.05, ** p < 0.01, *** p < 0.001, ## p < 0.001 (comparison between control cells after 2 h and 8 h tunicamycin treatment), n.s.—not significant; Control DMSO (174 cells/18 experiments), Control 2 h tunicamycin (97 cells/10 experiments), Control 8 h tunicamycin (120 cells/11 experiments), S1R KD DMSO (141 cells/15 experiments), S1R KD 2 h tunicamycin (67 cells/7 experiments), S1R KD 8 h tunicamycin (74 cells/8 experiments), BD1047 DMSO (93 cells/9 experiments), BD1047 2 h tunicamycin (80 cells/7 experiments), BD1047 8 h tunicamycin (100 cells, 9 experiments).
2.3. Sigma-1 Receptor Is Promoting Mitochondrial Hyperpolarisation and NADH Consumption during Early ER Stress
Next, we investigated mitochondrial membrane potential (Ψm) using tetramethylrhodamine methyl ester (TMRM), a cationic fluorescent dye that accumulates in mitochondria in a membrane potential-dependent manner (Figure S2a). Ψm serves as a marker for mitochondrial bioenergetic status and was increased after 2 h of tunicamycin treatment, and restored to control levels after 8 h of treatment (Figure 3a). The increase in Ψm after 2 h was significantly lower in S1R KD cells in comparison to control cells (Figure 3b,c). The effect of S1R antagonist BD1047 was comparable to that of the siRNA-mediated KD (Figure S2b,c). Similar to mitochondrial ATP data, Ψm emphasizes the importance of S1R for mitochondrial bioenergetics during the initial steps of ER stress.
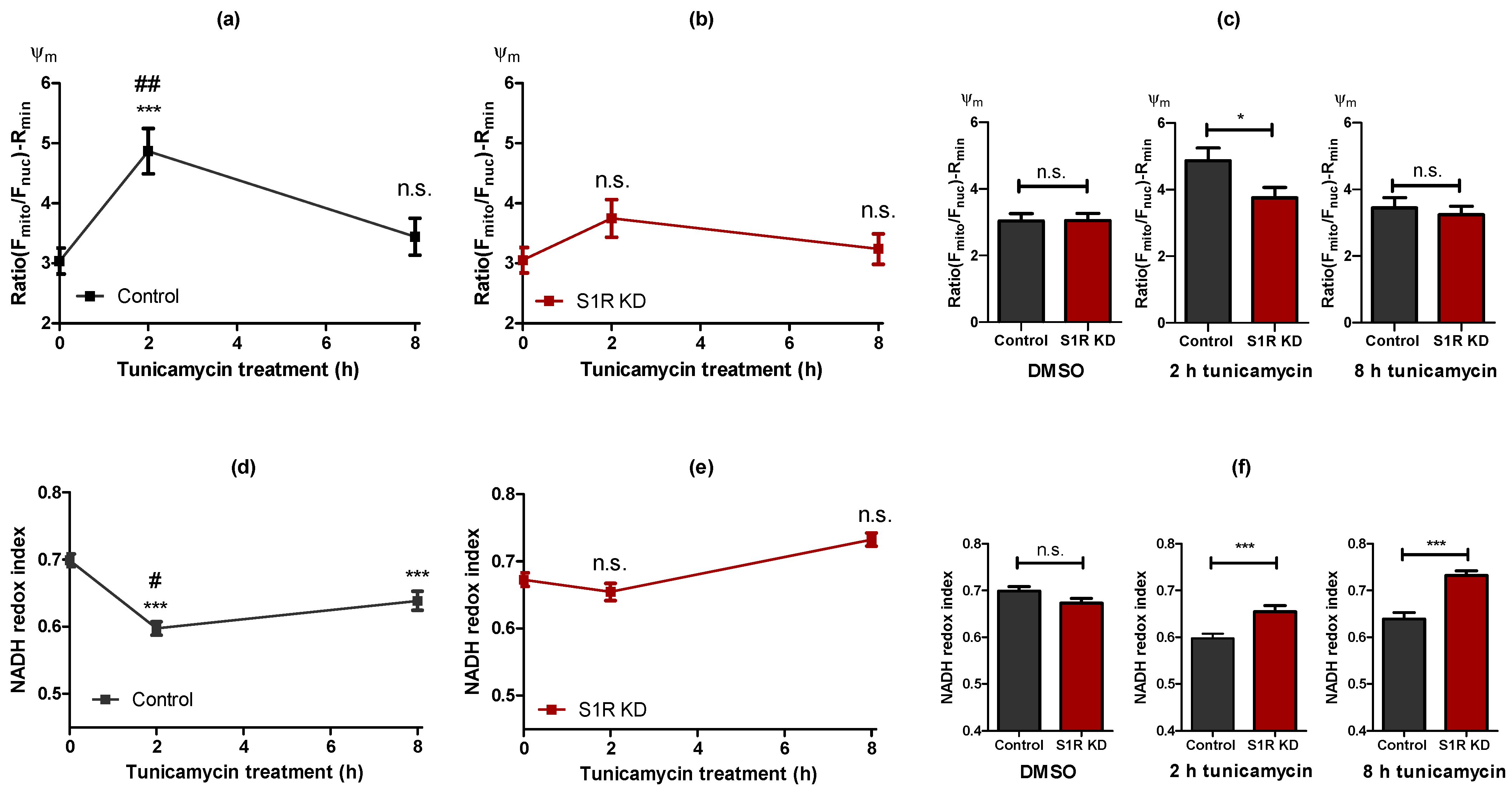
Alterations in the mitochondrial bioenergetic status are largely dependent on S1R during early ER stress. Time course of Ψm after tunicamycin treatment, presented as mean ± SEM and assessed by the mitochondrial to nucleus TMRM fluorescence ratio in control SH-SY5Y cells (a) and in cells with S1R KD (b). Bar graphs represent MEAN ± SEM of Ψm in control (black) and S1R KD (red) cells before tunicamycin treatment (left), after 2 h (middle), or 8 h (right) tunicamycin treatment (c). Time course of NADH redox index after tunicamycin treatment, presented as MEAN ± SEM and assessed NADH autofluorescence in control SH-SY5Y cells (d) and in cells with S1R KD (e). Bar graphs represent MEAN ± SEM of NADH redox index in control (black) and S1R KD (red) cells before tunicamycin treatment (left), after 2 h (middle), or 8 h (right) tunicamycin treatment (f). One-way ANOVA with Tukey’s multiple comparison test (a,b,d,e), *** p < 0.001, ## p< 0.01 and # p < 0.05 (control 2 h tunicamycin against control 8hr tunicamycin), n.s.—not significant; unpaired t-test (c,f), *** p < 0.001, * p < 0.05, n.s.—not significant; Ψm: Control DMSO (57 cells/10 experiments), Control 2hr tunicamycin (31 cell/5 experiments), Control 8hr tunicamycin (42 cells/7 experiments), S1R KD DMSO (54 cells/10 experiments), S1R KD 2 h tunicamycin (29 cells/5 experiments), S1R KD 8 h tunicamycin (40 cells/7 experiments); NADH redox index: Control DMSO (208 cells/9 experiments), Control 2 h tunicamycin (203 cell/6 experiments), Control 8 h tunicamycin (143 cells/6 experiments), S1R KD DMSO (243 cells/9 experiments), S1R KD 2hr tunicamycin (111 cells/5 experiments), S1R KD 8hr tunicamycin (129 cells/6 experiments).
We next evaluated mitochondrial NADH redox index [16] as an additional readout for mitochondrial bioenergetics, measured as mitochondrial NADH autofluorescence normalized to the minimum and maximum values achieved by uncoupling and blocking of NADH dehydrogenase complex, respectively. NADH redox index was decreased by 2 h of tunicamycin treatment and less so by 8 h of treatment (Figure 3d), which indicates more energized mitochondria. Since the NADH redox index represents NADH/NAD+ ratio, the decline of this ratio, combined with increased mitochondrial membrane potential and ATP, means more flux of electron donors (NADH) to the electron transport chain (ETC). S1R KD abolished this reduction of the NADH redox index at both time points (Figure 3e). The difference between the control group and the S1R knock-down was only apparent during ER stress induction by tunicamycin treatment (Figure 3f).Since tunicamycin-induced alterations in mitochondrial ATP, Ψm, and NADH redox index were abolished by S1R KD and/or the usage of an S1R antagonist, we assume S1R to serve as a key player in controlling mitochondrial bioenergetics during ER stress. Hence, because the 2 h treatment with tunicamycin resulted in a pronounced boost in mitochondrial bioenergetics, and this increase is approaching baseline status after 8 h of ER stress, S1R seems to be crucial, particularly during the early phase of ER stress.
2.4. Early ER Stress Gives Rise to an Increased ER Ca2+ Flux That Is Directed towards Mitochondria by Sigma-1 Receptor
To clarify the mechanism of how S1R is facilitating the increase of mitochondrial bioenergetics during early ER stress, we investigated mitochondria-associated ER membranes (MAMs). Although increased MAMs were reported for HeLa cells after 4 h of ER stress [5], we observed no change in the MAMs of tunicamycin treated control cells or the respective S1R knock-down cells (Figure S3a,b). Further on, we investigated whether ER stress is associated with an ER Ca2+ leak by using genetically-encoded ER Ca2+ probe D1ER [17]. Thereby, the ER Ca2+ leak was estimated by the change in ER Ca2+ level in response to the removal of extracellular Ca2+, which excludes the possibility of ER Ca2+ refilling. ER Ca2+ leak was increased by tunicamycin treatment for 2 and 8 h in control cells (Figure 4a). Interestingly, ER Ca2+ leak remained unaffected by tunicamycin treatment in S1R KD (Figure 4b), and BD1047 treated (Figure 4c) cells, but was found to be elevated in untreated S1R KD and BD1047 cells compared to controls (Figure 4d, left panel). In addition to the ER Ca2+ leak, we observed an unexpected increase in basal ER Ca2+ load with 2 and 8 h of tunicamycin treatment (Figure S4a). Similar to the leak, basal ER Ca2+ of S1R KD cells did not change with tunicamycin treatment (Figure S4b) and was higher in non-treated S1R KD cells compared to controls (Figure S4d, left panel), whereas the basal ER Ca2+ of BD1047 treated cells did not differ from controls (Figure S4c,d).
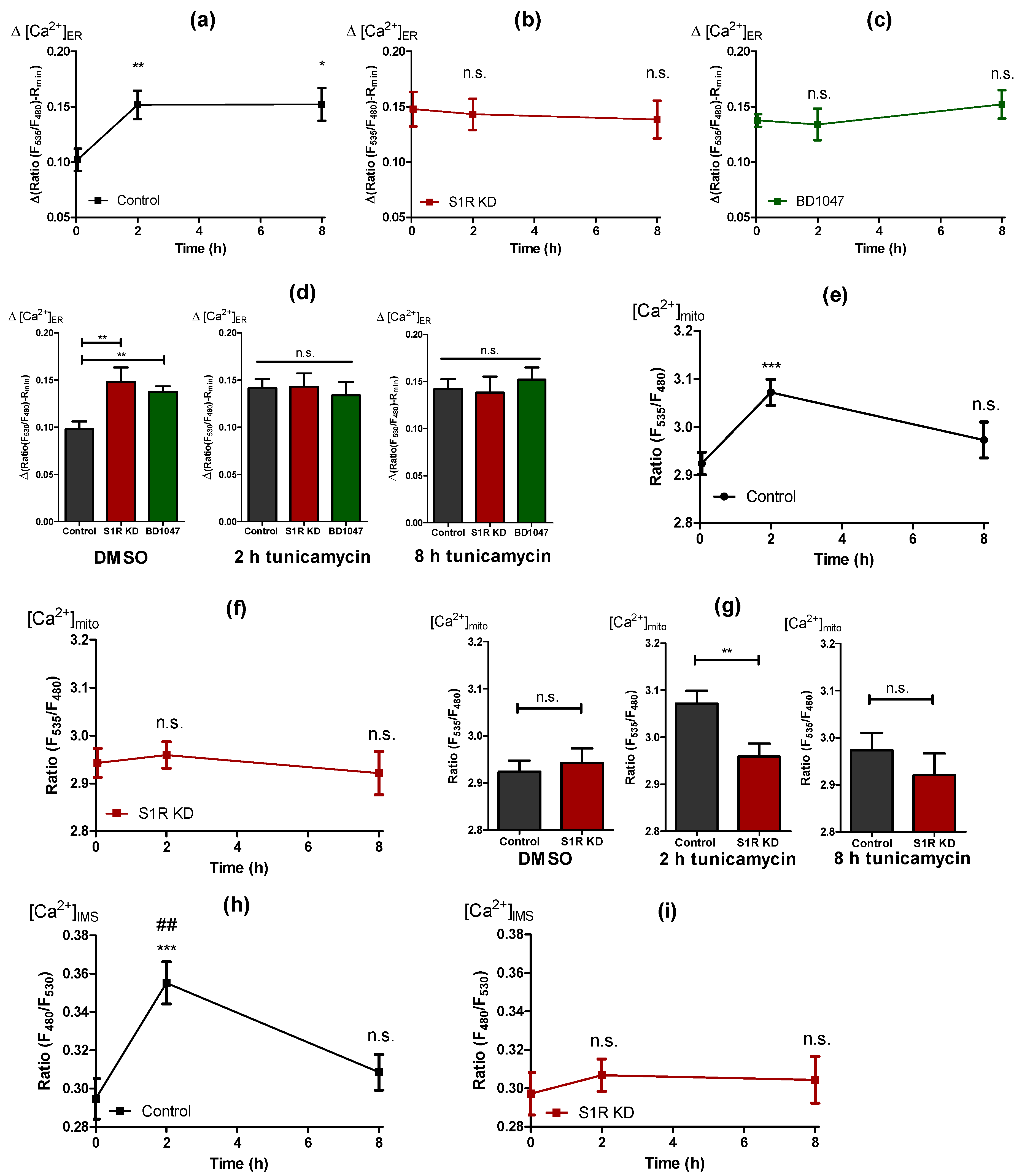
Cells develop an enhanced ER Ca2+ leak that is directed towards mitochondria by S1R during early ER stress. Time course of ER Ca2+ leak after tunicamycin treatment, presented as mean ± SEM and assessed by change in normalized D1ER ratio in control (a), S1R KD (b) and BD1047 treated (c) SH-SY5Y cells. Bar graphs represent MEAN ± SEM of ER Ca2+ leak in control (black), S1R KD (red) and BD1047 treated (green) cells before tunicamycin treatment (left), after 2 h (middle), or 8 h (right) tunicamycin treatment (d). Time course of mitochondrial Ca2+ level after tunicamycin treatment, presented as MEAN ± SEM and assessed by 4mtD3cpv ratio in control SH-SY5Y cells (e) and in cells with S1R KD (f). Bar graphs represent MEAN ± SEM of mitochondrial Ca2+ levels in control (black) and S1R KD (red) cells before tunicamycin treatment (left), after 2 h (middle), or 8 h (right) tunicamycin treatment (g). Time course of IMS Ca2+ level after tunicamycin treatment, presented as MEAN ± SEM and assessed by IMS-GEM-GECO ratio in control SH-SY5Y cells (h) and in cells with S1R KD (i). Bar graphs represent MEAN ± SEM of IMS Ca2+ levels in control (black) and S1R KD (red) cells before tunicamycin treatment (left), after 2 h (middle), or 8 h (right) tunicamycin treatment (j). One-way ANOVA with Tukey’s multiple comparison test (a–c,e,f,h,i), *** p < 0.001, ** p < 0.01, * p < 0.05, ## p < 0.01 (for h, control 2 h tunicamycin against control 8 h tunicamycin), n.s.—not significant; unpaired t-test (d,g,j), *** p < 0.001, ** p < 0.01, * p < 0.05, n.s.—not significant; ER Ca2+ leak: Control DMSO (43 cells/15 experiments), Control 2 h tunicamycin (44 cell/16 experiments), Control 8 h tunicamycin (38 cells/12 experiments), S1R KD DMSO (19 cells/9 experiments), S1R KD 2 h tunicamycin (27 cells/10 experiments), S1R KD 8 h tunicamycin (19 cells/6 experiments), BD1047 DMSO (23 cells/11 experiments), BD1047 2 h tunicamycin (9 cells/6 experiments), BD1047 8 h tunicamycin (18 cells/6 experiments); Mitochondrial Ca2+: Control DMSO (38 cells/14 experiments), Control 2 h tunicamycin (40 cell/13 experiments), Control 8 h tunicamycin (29 cells/8 experiments), S1R KD DMSO (35 cells/14 experiments), S1R KD 2 h tunicamycin (38 cells/12 experiments), S1R KD 8 h tunicamycin (25 cells/8 experiments); IMS Ca2+: Control DMSO (37 cells/4 experiments), Control 2 h tunicamycin (35 cell/4 experiments), Control 8 h tunicamycin (28 cells/3 experiments), S1R KD DMSO (38 cells/4 experiments), S1R KD 2 h tunicamycin (39 cells/4 experiments), S1R KD 8 h tunicamycin (36 cells/4 experiments).
The increase in ER Ca2+ leak in response to tunicamycin could explain increased mitochondrial bioenergetics in control cells, since the increased leak would supply mitochondria with more Ca2+ under these conditions. However, since a similarly strong ER Ca2+ leak was also established by S1R knock-down and BD1047 treatment, but without changes in mitochondrial bioenergetics, further experiments were necessary to clarify these differences. Thus, we also quantified basal mitochondrial Ca2+ level using the genetically encoded mitochondria-targeted Ca2+biosensor, 4mtD3cpv [18]. We observed increased mitochondrial Ca2+ levels in control cells in response to tunicamycin treatment (Figure 4e). Interestingly, mitochondrial Ca2+ levels were not significantly affected by S1R knock-down cells with or without tunicamycin treatment (Figure 4f,g) despite the increased ER Ca2+ leak (Figure 4b,d). These results point to a possible function of S1R during early ER stress, which is directing the enhanced ER Ca2+ leak towards sites of mitochondrial Ca2+ uptake, while the knock-down of S1R yields an undirected ER Ca2+leak, which is less sensed by mitochondria. The fact that ER Ca2+ leak was not changed between 2 h and 8 h of tunicamycin treatment (Figure 4a), but mitochondrial Ca2+ level is diminished from 2 h to 8 h of ER stress (Figure 4e), points to a possibility that S1R’s “directing” activity of ER Ca2+ leak is a time-dependent phenomenon. The diminishing of the mitochondrial Ca2+ increase after 8 h of ER stress also explains the return of mitochondria to the basal bioenergetic state observed in control cells (Figure 2a and Figure 3a,d). To test whether the observed differences in mitochondrial Ca2+ were due to the direction of the ER Ca2+ leak and not associated with Ca2+ “permeability” of the mitochondrial inner membrane, we measured Ca2+ levels in the mitochondrial inter-membrane space (IMS) using a recently developed IMS-targeted genetically encoded ratiometric Ca2+ sensor, IMS-GEM-GECO [19,20]. IMS Ca2+ closely followed the same trends as the mitochondrial Ca2+, with an increase after 2 h of tunicamycin treatment, which was normalized after 8 h of treatment (Figure 4h and Figure S5a). In S1R depleted and BD1047 treated cells, tunicamycin treatment did not elevate IMS Ca2+ after 2 h and 8 h (Figure 4i,j and Figure S5b,c), thus supporting our assumption of a specific role of S1R as an orchestrator of the enhanced ER Ca2+ leak during early ER stress, directing the Ca2+ leak towards mitochondrial Ca2+ uptake sites.
2.5. Sigma-1 Receptor Protects against Increased Mitochondrial ROS Production during ER Stress
Along with Ca2+ signaling and energy metabolism, mitochondrial reactive oxygen species (ROS) production plays a significant part in mitochondrial and cellular fitness under ER stress [21,22]. As it has been reported that S1R has an important role in mitochondrial ROS metabolism [8,23], we investigated the contribution of S1R to mitochondrial ROS production in our model of early ER stress in SH-SY5Y neuroblastoma cells. We monitored mitochondrial ROS using the genetically encoded mitochondrial ROS sensor, mitoHyper7 [24]. Induction of ER stress in control cells with tunicamycin for 2 h did not yield a change in mitochondrial ROS, whereas after 8 h of treatment, mitochondrial ROS levels significantly increased (Figure 5a). On the other hand, tunicamycin treatment resulted in a reasonably linear increase in mitochondrial ROS in S1R knock-down cells, with drastically increased ROS levels after 8 h of treatment (Figure 5b). Basal mitochondrial ROS, as well as ROS levels after 2 h and 8 h of tunicamycin treatment, were increased by S1R knock-down (Figure 5c). These results indicate that S1R prevents mitochondrial ROS production during early ER stress.
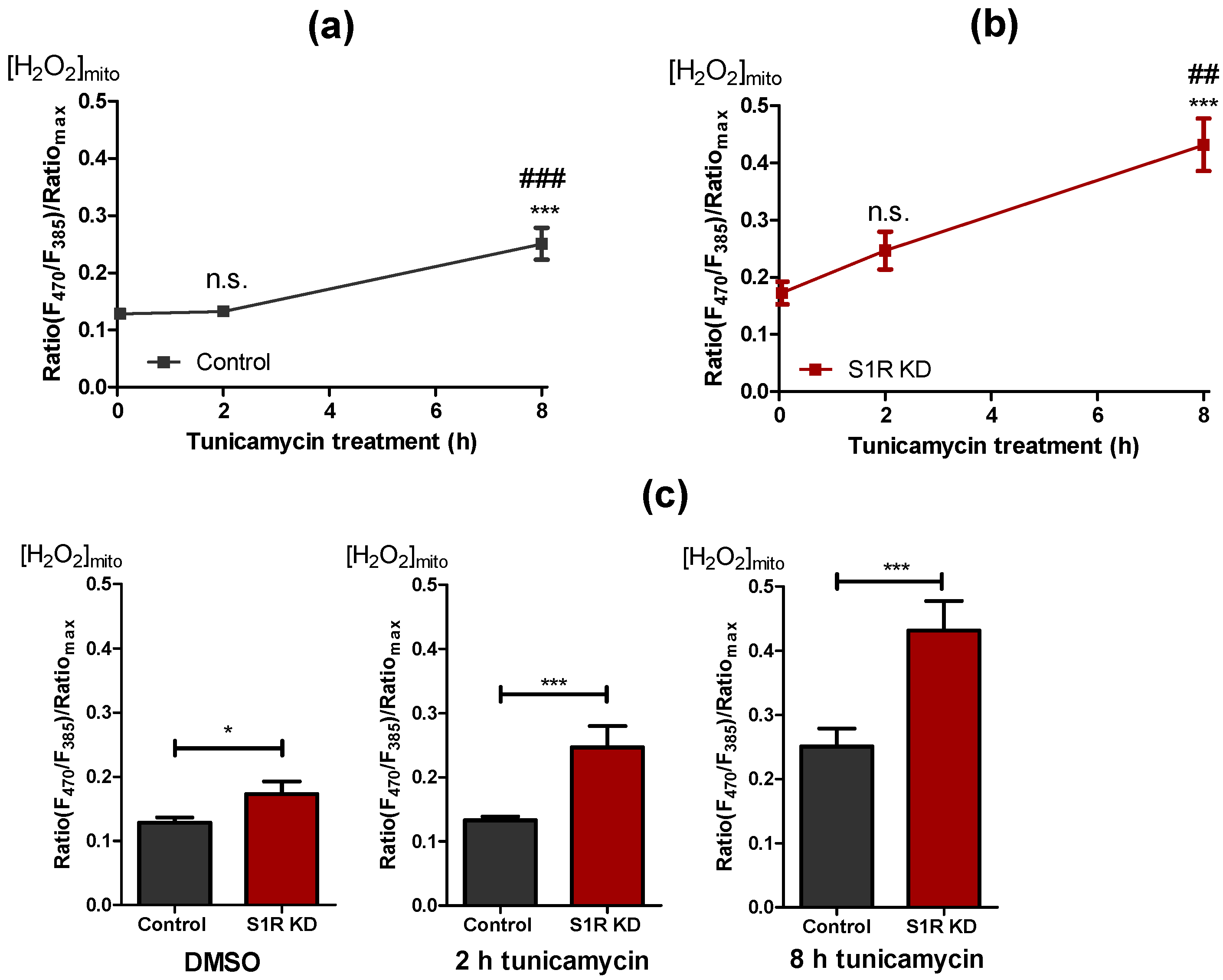
S1R KD is accompanied by increased ROS levels. Time course of mitochondrial H2O2 levels after tunicamycin treatment, presented as mean ± SEM and assessed by the mitoHyPer7 in control SH-SY5Y cells (a) and in cells with S1R KD (b). Bar graphs represent mean ± SEM of mitochondrial H2O2 levels in control (black) and S1R KD (red) cells before tunicamycin treatment (left), after 2 h (middle), or 8 h (right) tunicamycin treatment (c). Comparison of the mitochondrial ROS level in control and S1R KD cells at corresponding treatment durations (error bars are SEM); One-way ANOVA with Tukey’s multiple comparison test (a,b), *** p < 0.001, ## p < 0.01 (for b, S1R KD 8 h against S1R KD 2 h), ### p < 0.001 (for a, control 8 h against control 2 h), n.s.—not significant; unpaired t-test (c), * p < 0.05, *** p < 0.001; Control DMSO (22 cells/7 experiments), Control 2 h tunicamycin (32 cell/10 experiments), Control 8 h tunicamycin (22 cells/6 experiments), S1R KD DMSO (19 cells/9 experiments), S1R KD 2 h tunicamycin (27 cells/10 experiments), S1R KD 8 h tunicamycin (19 cells/6 experiments).
3. Discussion
Since ER stress and UPR are hallmarks of many human pathologies, including neurodegeneration and cancer [9,11], we have attempted to clarify the involvement of S1R in mitochondrial bioenergetics during the early phases of ER stress. We have induced ER stress in SH-SY5Y neuroblastoma cells with tunicamycin treatment for 2 and 8 h and validated it by quantifying XBP1 splicing (Figure 1a,b). Our data did not support previously reported findings that S1R affects XBP1 splicing [8], which is likely explained by the low knock-down efficiency of S1R in our model system (Figure S1a). Low knock-down efficiency is stemming out of a low transfection rate (10%) and the absence of a marker for transfected cells in the qPCR analysis used to quantify XBP1 splicing. When the knock-down efficiency was assessed in co-transfected GFP-positive sorted cells, S1R mRNA level was reduced by 75% (Figure S1b). Consequently, the absence of a significant difference in XBP1 splicing between control and S1R knock-down cells is not definitive. A slight reduction of XBP1 splicing in S1R KD cells was observed after 8 h of tunicamycin treatment and might indicate the reduced IRE1 activity because of S1R knock-down. For all the remaining experiments, we have inclined towards single-cell measurements. Having established ER stress induction over time in vitro, we could demonstrate that mitochondrial bioenergetics are getting substantially augmented after 2 h of ER stress (Figure 2a and Figure 3a,d). S1R seems to play a major role in this adaptation, since knock-down and a pharmacological antagonist of S1R almost eliminated respective responses (Figure 2b,c and Figure 3b,e and Figure S2c). In search for a possible mechanism of action of S1R, we measured the amount of MAMs, as it has been reported that MAMs are increasing during early or later stages of ER stress [5,25]. Our results in SH-SY5Y cells showed no detectable changes in MAMs after 2 h and 8 h of tunicamycin treatment in control or S1R knock-down cells (Figure S3a,b). The possible reasons for discrepancies with published data could be specificities of cell lines used, as most of the reported studies were performed in HeLa cells [5,25]. Next, we looked directly into ER Ca2+ leak, since it can influence mitochondrial energetics during ER stress and was previously reported to be increased during ER stress [26,27]. In line with these reports, we have observed increased ER Ca2+ leak after 2 h and 8 h of tunicamycin treatment in control cells (Figure 4a). Still, surprisingly, a similar leak was present in untreated S1R knock-down and BD1047 treated cells, which did not change with tunicamycin treatment (Figure 4b–d). This was a puzzling finding, since the increased leak in S1R KD and BD1047 treatment did not fit together with mitochondrial energetics data, unless the Ca2+ leak was not directed towards mitochondria in knock-down BD1047 treated cells.We could elaborate supportive data for the latter as mitochondrial, and IMS Ca2+ levels were increased after 2 h of tunicamycin treatment in control and not in S1R KD and antagonist treated cells (Figure 4e–j and Figure S5a–c), despite the presence of comparable ER Ca2+ leak after 2 h and 8 h of ER stress in all conditions. The prevalence of the increased Ca2+ level in the IMS during early ER stress in control and not in S1R KD and BD1047 treated cells validates the driving role of S1R directed ER Ca2+ leak as a promoter of mitochondrial bioenergetics and removes the possibility of hampered permeability of mitochondrial inner membrane as a result of reduced level or activity of S1R. Additionally, since the reduction of S1R expression by siRNA or the activity by the antagonist on its own was enough to trigger ER Ca2+ leak (Figure 4d, left panel) that was not directed to mitochondria, it is easy to speculate that S1R is essential for ER homeostasis under unstressed conditions as well.In support of our findings, a recent study elegantly showed a UPR-independent function of IRE1, where it serves as a scaffold for IP3R at the MAMs [28], and another study showed that S1R stabilizes IRE1 during ER stress at the MAM region [8]. Together with these interesting reports, our current work points towards the function of S1R as a director of ER Ca2+ towards mitochondria during early ER stress as a mechanism to increase mitochondrial bioenergetics to eventually supply more ATP for UPR. Diminishing mitochondrial ATP and bioenergetics after 8 h of ER stress (Figure 2a and Figure 3a,d) despite the same enhanced ER Ca2+ leak (4a) in control cells adds an extra argument in support of time dependency of S1R orchestrated ER Ca2+ leak directed at mitochondria. In support of this claim, it was previously shown that S1R redistributes from the MAM region towards the remaining parts of the ER after prolonged ER stress [7], hence its ER leak directing function would be decreasing as ER stress progresses further. As a result, the ER Ca2+ leak would no longer be pointed towards mitochondria, as evidenced by a drop of the mitochondrial and IMS Ca2+ levels back to basal levels after 8 h of ER stress (Figure 4e,h).Although the matter of ER Ca2+ leak directed towards sites of mitochondrial Ca2+ uptake by S1R to enhance mitochondrial bioenergetics to supply ATP for ER UPR seems fitting to the overall picture, the molecular identity of protein(s) responsible for the S1R controlled mitochondria-directed ER Ca2+ leak is not clear. We are tempted to speculate that IP3R3 is the likely candidate, but this claim needs further investigation. Our findings of increased basal ER Ca2+ levels that we observed in S1R knock-down cells and control cells treated with tunicamycin are not clear and need further attention. One possible explanation might be that S1R antagonizes store-operated Ca2+ entry (SOCE) [29]; hence the knock-down of S1R might result in increased ER Ca2+ level as a result of increased SOCE. Additionally, increased mitochondrial ATP production during early ER stress (Figure 2a) could enhance ER Ca2+sequestration through sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA) activity.By increasing mitochondrial bioenergetics during early ER stress, S1R maintains a supply of ATP to fuel UPR to restore ER homeostasis, but in the meanwhile, it is known that enhanced oxidative phosphorylation can generate ROS. To compensate for this, S1R shows the ability to moderate excessive ROS production, as we have demonstrated S1R’s importance in maintaining a balanced ROS metabolism under resting and ER stress conditions (Figure 5). These findings are supported by published data emphasizing the involvement of S1R in protection against oxidative stress and provide a possible mechanism for enhanced cell death of S1R deficient cells undergoing ER stress [7,8,23]. Taken together, by simultaneously enhancing mitochondrial bioenergetics and reducing ROS generation, S1R acts as a pro-survival agent for a cell facing ER stress. In conclusion, we have demonstrated that S1R plays a crucial role during the early stages of ER stress, whereby it promotes mitochondrial bioenergetics by directed Ca2+ mobilization and protects against mitochondrial ROS elevation. We provide novel mechanistic insights into the complex ER-to-mitochondria communication during the onset of ER stress which might have multiple implications in different human pathologies, and highlight the need for further studies involving pre-clinical disease models involving ER stress, such as Alzheimer’s disease and other neurodegenerative disorders.
…

