Safe and Efficient Sigma1 Ligand: A Potential Drug Candidate for Multiple Sclerosis
By Bénédicte Oxombre, Fahima Madouri, Anne-Sophie Journé, Séverine Ravez, Eloise Woitrain, Pascal Odou, Nathalie Duhal, Sandro Ninni, David Montaigne, Nadira Delhem, Patrick Vermersch, and Patricia Melnyk
Excerpt from the article published in International Journal of Molecular Sciences 23, no. 19: 11893. 6 October 2022. DOI: https://doi.org/10.3390/ijms231911893
Editor’s Highlights
- In the last few decades, the sigma1 (S1R) ligand-regulated chaperone has emerged as a promising new target in Multiple Sclerosis (MS).
- Eliprodil, an NMDA receptor antagonist with S1R affinity, has been shown to promote myelination in neuron–oligodendrocytes coculture.
- In an experimental model of MS, S1R can indeed be found in monomeric and oligomeric forms in physiological conditions, and it has been shown that agonists favor monomers and/or dimers while antagonists favor tetramers, hexamers, octamers and perhaps even higher order oligomers.
- A single i.p. injection of the S1R agonist 1(S), decreased the clinical progression of EAE disease and prevented mononuclear cell accumulation and demyelination in the brain and spinal cord.
- S1R agonists may also be useful for adjunct treatment of MS and/or further development in progressive MS due to their manifold properties.
- Boosting S1R activity leads to intracellular calcium homeostasis restoration, the stabilization of mitochondrial physiology and cellular adaptive response facilitation.
Abstract
Multiple Sclerosis (MS) is an autoimmune demyelinating and neurodegenerative disease of the central nervous system (CNS). Current management strategies suppress or modulate immune function, all with consequences and known side effects. They demonstrate a high level of success in limiting new relapses. However, the neurodegenerative process still affects both grey and white matter in the central nervous system. The sigma1 (S1R) ligand-regulated chaperone is implicated in many biological processes in various CNS-targeted diseases, acting on neural plasticity, myelination and neuroinflammation. Among the proteins involved in MS, S1R has therefore emerged as a promising new target. Standard and robust methods have been adopted to analyze the adsorption, distribution, metabolism, excretion (ADME) properties, safety pharmacology and toxicology of a previously synthetized simple benzamide-derived compound with nanomolar affinity for S1R, high selectivity, no cytotoxicity and good metabolic stability. The compound was also characterized as an agonist based on well-validated assays prior to in vivo investigations. Interestingly, we found that the oral administration of this compound resulted in an overall significant reduction in clinical progression in an MS experimental model. This effect is mediated through S1R action. Our results further suggest the potential use of this compound in the treatment of MS.
1. Introduction
New drugs targeting the central nervous system (CNS) have been increasingly studied and characterized over the span of the last half-century. One of the biggest challenges faced in preclinical trials of CNS-targeted disease is that drugs have to cross through the blood brain barrier (BBB). It is also mandatory to establish an appropriate balance between drug efficacy and potential adverse effects in the early stages of evaluation. In Alzheimer’s disease (AD), the ABCDE paradigm has been recently defined as a conceptual tool to facilitate the development of safe and effective therapies that can be summed up as being (A) accessible, (B) BBB permeant, (C) associated with improvements in clinical symptoms, (D) disease-modifying and (E) environmentally nontoxic [1]. This ABCDE concept is in fact applicable to all CNS diseases. The chemical structure of a drug is indicative of its physicochemical properties (solubility, stability, etc.), and its drug-like properties such as adsorption, distribution, metabolism, excretion (ADME) and toxicity, also including metabolic stability, plasma stability, P-glycoprotein (P-gp) extrusion, serum albumin binding, cytochrome P450 (CYP) inhibition or human Ether-à-go-go Related Gene (hERG) inhibition. These characteristics affect drug bioavailability and pharmacodynamic activity, which forecast its potential clinical achievements. Preclinical studies essentially enable us to define these parameters and assess whether a new drug could be a moderate or even low risk candidate while being considered for entering a clinical trial.
Multiple sclerosis (MS) is the leading cause of non-traumatic neurological disability arising in young adults. MS is characterized by the pathological hallmarks of inflammation with demyelination, astrogliosis and neurodegeneration. Tissue damage in MS is restricted to the CNS and MS symptoms vary according to the location and severity of the lesions. MS can also present a relapsing or progressive evolution, but the same underlying disease process remains engaged. Inflammation is typically associated with relapses while neurodegeneration is associated with the progression of MS. Current therapeutic strategies are focused on managing the inflammatory component. Several monoclonal antibody therapies have been developed in the past decade [2]. B-cell depleting strategies demonstrate high success in limiting new relapses and the accumulation of focal lesions using magnetic resonance imaging. The impact of currently approved drugs is limited on disability in the progressive forms of MS [3]. Despite these drugs exhibiting many beneficial effects, some serious adverse effects (AE) have been reported in many experiments and clinical trials [4] and reparative approaches are still needed. In addition, biologics are relatively complex molecules to develop. One current research strategy is the development of small molecule drugs that can be administered by a variety of routes, especially orally [5]. The neurodegenerative process occurs early on in both gray and white matter of the CNS, and may clearly play a significant role in the first stages of MS. Mitochondrial dysfunction is considered to be one of the main contributors to axonal damage [6]. In fact, mitochondrial homeostasis is essential in maintaining proper energy production and calcium regulation, both of which are crucial functions in the healthy functioning of neurons and oligodendrocytes.
The sigma opioid receptor was described for the first time in 1976 by Martin et al. but later recognized as sigma receptor by Su TP [7,8]. The two subtypes of sigma receptors were identified as sigma1 (S1R) and sigma2 (S2R) receptors, and S1R was successfully cloned in 1996 [9]. Anatomically, S1R is expressed in peripheral organs such as the kidney, liver and lung, but our interest resides in the fact that S1R is widely distributed in numerous brain areas. Numerous studies indicate the role of S1R in neurological disorders such as schizophrenia, depression and anxiety, addiction and alcoholism, as well as in neurodegenerative disorders [10,11,12]. Cellular studies have shown that S1R is located on the endoplasmic reticulum (ER)/mitochondria interface, in a region called the mitochondria-associated ER membrane (MAM), and that it is implicated in proper Ca2+ signaling between the ER and mitochondria [13,14]. Under normal resting conditions, S1R forms a complex with another chaperone, the glucose-regulated protein GRP78, also known as BiP. Under pathological/stressful conditions, the Ca2+ concentration at the ER dramatically drops and S1R dissociates from BiP. Studies also indicate that S1R translocates to other subcellular compartments when cells are stimulated or when undergoing prolonged stress. Functionally, S1R is not a typical pharmacological receptor, as no transduction systems have been identified [15]. S1R has been characterized as a unique ligand-operated molecular chaperone [16] that can amplify or reduce the signaling incurred upon activation of several receptors, ion channels, transporters and enzymes, and thus is involved in the modulation of different physiological functions [17,18]. As a ligand-regulated chaperone, the modulatory activity of S1R can be augmented or inhibited. S1R binds diverse classes of pharmacological ligands. Some of the major high-affinity S1R ligands function as pharmacological agonists or antagonists and result in physiological response. There are also allosteric modulatory ligands that have no pharmacological activities by themselves but which elicit a positive or negative modulatory effects when certain physiological processes are engaged [19]. It must also be considered that S1R does not evoke an effect under normal physiological conditions, but results in marked cellular effects caused by being activated in stressful conditions [20,21]. In the CNS, S1R contributes to many biological processes and S1R compounds may offer promise as a disease-modifying pharmacological target in various CNS-targeted diseases by acting on neural plasticity, myelination and neuroinflammation [11,22,23]. Among the proteins involved in MS, S1R has therefore emerged as a promising new target [12,24].
Since its discovery, significant progress in the understanding of S1R function has been achieved. Pharmacophore modeling and crystal structures have provided knowledge essential in understanding its modulation. Extensive drug discovery campaigns have provided the development of small chemical molecules targeting S1R [25]. In our laboratory, tetrahydroisoquinoline-hydantoin has been described as a key pharmacophore [26] and our previous study evidenced that a single injection of one high-affinity and selective S1R agonist with a tetrahydroisoquinoline-hydantoin scaffold reduces the clinical progression of disease. This has been observed in an MS experimental model through the prevention of mononuclear cell accumulation and demyelination in the brain and spinal cord [27]. Nevertheless, this compound showed low metabolic stability. Therefore, a novel series of benzamide-derived compounds were designed, synthetized and pharmacologically evaluated [28]. Many S1R ligands have mixed binding affinity for both S1R and S2R, as well as other receptor types; however, compound 7i showed excellent affinity for S1R (Ki = 3.2 nM) and selectivity for S2R (Ki up to 1400 nM) with a high selectivity index (Table 1 and Table 2). Undesirable off-target effects were identified early using pharmacological profiling against 40 different receptors relevant to CNS disease, which indicated that compound 7i had an excellent highly selective profile. Compound 7i also presented no cytotoxicity. It is also interesting because the physical properties of compound 7i are also favorable for drug development.
| nLM | hLM | ||||||
|---|---|---|---|---|---|---|---|
| t1/2 (h) | CLint (µL/min/pmol) | Compound Remaining (1 h, %) | t1/2 (h) | CLint (µL/min/pmol) | Compound Remaining (1 h, %) | ||
| 7i | 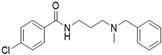 | 1.2 | <115 | 54 | 1.9 | <115 | 66 |
| 7i-deMe |  | 3.9 | <115 | 80 | 10.0 | <115 | 83 |
Metabolic stability profiles. Microsomal stability of compounds 7i and 7i-deMe was assessed in mouse (mLM) and human (hLM) liver microsomes at 10 µM. Half-life (t1/2) and intrinsec clearance (CLint) were evaluated. % compound remaining after 1 h was determined.
| Ki | Inhibition | |||
|---|---|---|---|---|
| S1R (nM) | S2R (nM) | Ratio S2R/S1R | SH-SY5Y (% at 100 µM) | |
| 7i [28] | 3.2 | 190 | 60 | 28 |
| 7i-deMe | 23 | >1000 | >500 | 77 |
S1R and S2R affinities and the cytotoxicity of compounds 7i [28] and 7i-deMe.
In the present study, an extensive in vitro analysis was used in order to establish as many arguments as possible to show that compound 7i exhibits ideal characteristics that allow it to be considered a good drug candidate for CNS-targeted diseases. The impact of compound 7i was then analyzed in vivo to validate that the new drug presents a moderate risk for clinical application and that S1R modulation significantly reduces clinical progression of the disease in an MS experimental model.
2. Results
2.1. In Vitro Drug Candidate Assessment
2.1.1. Metabolic Stability and Metabolite Identification and Profiling
Kinetic study of the microsomal stability of compound 7i was performed in mouse and human liver microsomes (i.e., SER membrane re-form into vesicles) at 10 µM (Table 1). Compound 7i shows a half-life greater than 1 h and exhibits good clearance (<115 µL/min/pmol). After 1-h incubation, only 20% of compound 7i is metabolized and detected in demethylated form (named hereafter as 7i-deMe, Figure S1). Demethylation slightly decreases S1R affinity (23 nM), and more drastically S2R affinity (>1000 nM), increasing the S2R/S1R ratio by up to 500 (Table 2). The potential cytotoxic effects of compound 7i-deMe were analyzed on a human neuroblastoma cell line at different concentrations up to 100 µM. Compound 7i-deMe exhibits higher cytotoxicity than compound 7i [28]. Compound 7i-deMe shows a half-life greater than 3 h in mouse liver microsomes and 9 h in human liver microsomes (Table 1). No modification of clearance is observed. Its affinity for S1R, its selectivity and its low cytotoxicity make it a compound comparable to compound 7i.
2.1.2. Preliminary ADME Studies
Compound 7i was then evaluated for in vitro properties essential for later stages of drug development. In the first step, preliminary ADME study was focused on bioavailability features at 10 µM (Table 3). As expected, compound 7iexhibited high solubility in PBS solution at pH 7.4 (185 µM). Solubility and stability were not modified using simulated gastric fluid (SGF) and intestinal fluid (SIF), mimicking digestion. Permeability across cell membranes was assessed to investigate intestinal permeability and predict the absorption of orally administered drugs using apical (A, pH 6.5) and basolateral (B, pH 7.4) chambers representing the luminal and blood/mesenteric lymph sides of the gastrointestinal tract, respectively. Compound 7i shows good A/B permeability (up to 20 × 10−6 cm/s) and an efflux ratio close to 2. Its stability in plasma lasts greater than 24 h. Compound 7i affinity to plasma protein is below 95%. In the liver, CYP enzymes are important metabolic players. At the biochemical level, CYP families 1 to 3 constitute major enzymes for the biotransformation of small molecule drugs [29]. Therefore, CYP inhibition analysis was performed on human 1A, 2B6, 2C8, 2C9, 2C19, 2D6 and 3A as recommended by FDA and EMA guidance. No to weak inhibition was observed, except for CYP2D6. Membrane transport proteins can also influence the pharmacokinetics of many drugs and may be implicated in drug–drug interactions. They are categorized into ATP-binding cassette (ABC) and solute-linked carrier (SLC) families and are expressed by several tissues, such as the intestine, liver, kidney and brain [30]. In a third step, the inhibitory effects of compound 7i were evaluated in five ABC (P-gp, BCRP, MRP1, 2 and 3) and eight SLC (OATP1B1 and 3, OAT1 and 3, OCT1 and 2, ABST and NTCP) transporters. The inhibitory effects of compound 7i are low (highest values are below 50% inhibitory effect), showing that the compound has no membrane transporter inhibitor capacity. Membrane transporters also play a critical role in new molecular entity (NME) absorption through active transport. Metabolite 7i-deMe has similar characteristics to compound 7i (Table 3).
| 7i | 7i-deMe | |
|---|---|---|
| Solubility (µM) | ||
| PBSpH7.4 | 185 | 187 |
| SGF | 193 | 197 |
| SIF | 188 | 196 |
| Permeability (×10−6 cm/s) | ||
| A/B pH6.5/7.4 | 29.1 | 31.1 |
| B/A pH6.5/7.4 | 66.1 | 51.5 |
| e-ratio | 2.3 | 1.7 |
| Plasma t½ (h) | 25.9 | 23 |
| PPB (%) | 94 | 81 |
| CYPs inhibition (%) | ||
| CYP1A | 2.1 | 9.4 |
| CYP2B6 | −4.3 | 15.9 |
| CYP2C8 | −9.2 | −7.2 |
| CYP2C9 | −7.2 | 5.9 |
| CYP2C19 | 36.5 | 18.4 |
| CYP2D6 | 95.1 | 91.6 |
| CYP3A | 36.9 | 3.2 |
| Drug transporters inhibition (%) | ||
| ABC family | ||
| P-gp | 10.6 | 1.0 |
| BCRP | 8.3 | −3.0 |
| MRP1 | 1.9 | −3.4 |
| MRP2 | −18.8 | −11.5 |
| MRP3 | −0.3 | −0.6 |
| SLC family | ||
| OATP1B1 | 12.0 | 8.6 |
| OATP1B3 | 23.1 | 19.1 |
| OAT1 | −9.6 | −6.2 |
| OAT3 | 34.3 | 18.7 |
| OCT1 | 41.2 | 64.6 |
| OCT2 | 49.4 | 85.8 |
| ABST | 5.3 | 5.0 |
| NTCP | −1.7 | 8.7 |
| P-gp substrate (×10−6 cm/s) | ||
| PBS (×10−6 cm/s) | ||
| A/B pH7.4/7.4 | 45.8 | |
| B/A pH7.4/7.4 | 11.7 | |
| e-ratio | 0.3 | |
| Verapamil (×10−6 cm/s) | ||
| A/B pH7.4/7.4 | 28.0 | |
| B/A pH7.4/7.4 | 16.9 | |
| e-ratio | 0.6 | |
| CYPs induction (%) | ||
| CYP1A2 | ||
| #1 (2) | 2.7 | |
| #2 (5) | 1.5 | |
| #3 (2) | 1.9 | |
| CYP2B6 | ||
| #1 (3) | 4.1 | |
| #2 (4) | 4.1 | |
| #3 (2) | 4.2 | |
| CYP3A4 | ||
| #1 (4) | 4.2 | |
| #2 (6) | 1.6 | |
| #3 (3) | 0.8 |
Preliminary ADME study of compound 7i and major metabolite 7i-deMe.
Solubility was analyzed in PBS, simulated Gastric fluid (SGF) and simulated intestinal fluid (SIF). Efflux ratio (e-ratio), plasma half-life (t1/2) and plasma protein binding (PPB) were calculated. CYP and drug transporter inhibition, P-gp substrate and CYP induction were analyzed. CYP induction analysis was carried out on three donors whose individual cut-offs are noted in brackets. Analyses were performed at 10 µM. Results are expressed as mean (n = 2).
P-gp was identified as a major barrier protein for many CNS drug substances, and P-gp substrate activity remains a key selection target for CNS drug discovery [30]. Comparison of efflux ratios generated in the presence or absence of the P-gp inhibitor verapamil at pH 7.4 (0.3 and 0.6, respectively) indicates that compound 7i is not a substrate for P-gp transporter. Induction of the expression of CYPs can also dramatically alter the pharmacokinetics of a drug, its toxicity and impact drug–drug interactions [31]. CYP induction was evaluated on human hepatocytes (three donors). No modulation of CYP1A2, CYP2B6 or CYP3A4 was observed at 10 µM.
2.1.3. Safety Pharmacology and Toxicology
In vitro pharmacology profiling is increasingly being used earlier in drug discovery processes to identify undesirable off-target activity profiles that could hinder or halt the development of candidate drugs. Cardiac toxicity is one of the major reasons for drug failure. Early robust profiling panels are based on interaction with the well-characterized cardiac ion channel. Analysis was performed at three regulatory concentrations on the potassium voltage-gated channel subfamily H member 2 (KCNH2; also known as hERG) that is the only in vitro pharmacology assay absolutely required by regulatory authorities to study the effects of new chemical entities on ionic current [32]. Compound 7i was shown to inhibit hERG with an IC50 of 1 µM. Comparable results were obtained with compound 7i-deMe (IC50 of 1.2 µM). The cytotoxicity analysis of compound 7i was thorough and used human hepatocytes HepG2 cells (Table 4). No modifications in cell number, nuclear size, mitochondrial membrane potential, intracellular free calcium or membrane permeability were observed. Genotoxicity testing of drug candidates is also required to support clinical entry. The most common approach is to perform two separate in vitro assays (one by a bacterial reverse mutation and one in a mammalian cell) before any in vivo analysis. For in vitro mammalian cell testing, the micronucleus test (MN) is commonly used [33]. Compound 7i has no mutagenic effects, as shown by Ames tests on five bacterial strains (Table 5). The same results were obtained at six increasing concentrations (0.37 to 40 µg/plate). Table 6 shows the MN/2000-nuclei ratio and relative survival (RS) obtained in TK6 cells treated with compound 7i in two independent experiments. Cells treated with the positive controls show a significant increase in MN compared to the vehicle control cultures. Compound 7i shows no genotoxic characteristics.
| 7i | |
|---|---|
| Cardiotoxicity | |
| hERG inhibition (mM) | |
| IC50 | 1 |
| Cytotoxicity | |
| HepG2 (% at 10 µM) | |
| Cell number | −3 |
| Intracell free calcium | 0 |
| Nuclear size | 9 |
| Membrane permeability | 0 |
| Mitochondrial membrane potential | 5 |
Compound 7i cardiotoxicity and cytotoxicity analysis.
Results are expressed as mean (n = 2).
| TA1535 | TA1537 | TA98 | TA100 | TA102 | ||
|---|---|---|---|---|---|---|
| Dose (µg/plate) | −S9 | −S9 | −S9 | −S9 | −S9 | |
| Positive control | (a) | 85.9 | 8.4 | 23.1 | 13.5 | 6.0 |
| Vehicle control | 0 | – | – | – | – | – |
| 7i | 40 | 1.3 | 0.9 | 1.1 | 0.9 | 0.7 |
| +S9 | +S9 | +S9 | +S9 | +S9 | ||
| Positive control | (b) | 26.9 | 27.6 | 36.0 | 6.0 | 5.7 |
| Vehicle control | 0 | – | – | – | – | – |
| 7i | 40 | 0.9 | 1.1 | 1.1 | 0.9 | 1.0 |
Compound 7i genotoxicity analysis using the Ames test.
Five Salmonella S. typhimurium strains were used: TA1535, TA97a, TA98, TA100 and TA102 with (S9+) and without (S9−) external metabolic activation. The OECD 471 guideline was followed. Mean revertants/well of the technical triplicates in an experiment are shown. Positive controls (C+) are shown. (a) TA1535:MNNG 0.25; TA1537: 9-amino-acridine 1.56; TA98: 2-nitrofluorene 0.5; TA100: MNNG 0.25; TA102: Mitomycine 0.0625. (b) TA1535, TA1537, TA98, TA100:2-anthramine 0.5; TA102: Benzo(a)pyrene 4. Results are expressed as mean (n = 2).
| 3 h Short Treatment With 24 h Recovery Period | 27 h Continuous Treatment | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| −S9 | +S9 | −S9 | ||||||||
| µg/mL | RS (%) | MN/2 × 103 Cells | µg/mL | RS (%) | MN/2 × 103 Cells | µg/mL | RS (%) | MN/2 × 103 Cells | ||
| Positive control | Mytomice C | 0.5 | 62.8 | 153.0 | 0.2 | 67.9 | 62.0 | |||
| Griseofulvin | 5 | 70.5 | 63.0 | |||||||
| Cyclophosphamide | 5 | 88.3 | 35.5 | |||||||
| Vehicle control | 0 | – | 3.0 | 0 | – | 11.0 | 0 | – | 9.5 | |
| 7i | 275 | 80.0 | 8.0 | 275 | 103.7 | 9.0 | 275 | 70.1 | 6.0 | |
| 137.5 | 92.7 | 8.0 | 137.5 | 97.2 | 6.0 | 137.5 | 84.2 | 7.0 | ||
| 68.78 | 94.1 | 6.0 | 68.75 | 106.1 | 10.5 | 68.75 | 86.6 | 1.0 |
Compound 7i genotoxicity analysis with a micronucleus (MN) in TK6 cells.
Analysis was performed after 3-h treatment with DHA, rutin and α-tocopherol with (S9+) and without (S9−) metabolic activation and after 24 h without metabolic activation. MN per 103 nucleated cells and the relative survival rate (RS) of two independent experiments are shown. Results are expressed as mean (n = 2).
Chronic inflammation contributes to numerous diseases. Although it is not required to perform immunotoxicity assays prior to standard toxicology studies (STS) testing, we decided to analyze the impact of compound 7i on human peripheral blood mononuclear cells (hPBMCs) that were or were not stimulated with E. coli lipopolysaccharide (LPS) and αCD3/αCD28 for specific TCR activation. Lymphocyte subpopulations were immunophenotyped, including CD19 B, CD4 and CD8 T lymphocytes and natural regulatory T cells (Figure 1). On basal non-stimulating conditions, compound 7i has no effect on the percentage of human B, Th17 and T lymphocyte subpopulations (CD4 and CD8 T cells), nor regulatory T cells. As expected, in TCR stimulating conditions, a significant increase in activated CD4 and CD8 T lymphocytes has been observed, suggesting that compound 7i does not affect cellular immune adaptive systems and that immune function is preserved. This was confirmed by natural regulatory T cell prevalence that was also increased to naturally restore immune cell homeostasis. We also confirmed that compound 7i has no effect on Th17 cells that are known to induce an inflammatory microenvironment. Altogether, these results suggest that compound 7i has no toxicity effect on all immune subpopulations tested in stimulating or not stimulating conditions.

Immunotoxicity analysis. Human peripheral blood mononuclear cells (hPBMCs) were either non-stimulated (basal) or stimulated during the course of a 48-h period. Compound 7i was tested at 10 µM. Immune cell subpopulations were immunophenotyped among hPBMCs by flow cytometry. Cell percentage was evaluated. Data are presented as mean ± SEM from six individual healthy donors. * p < 0.05 vs. control (basal vehicle) condition. Wilcoxon–Mann–Whitney U test.
2.2. Evaluation of Compound 7i as a Sigma1 Receptor Agonist
S1R was historically classified as a receptor because a large number of high-affinity and selective small ligands triggered (for so-called agonists) or prevented (for so-called antagonists) biological responses. This understanding remains despite the fact that S1R’s mode of action relies on the modification of protein–protein interactions rather than coupling to second messenger systems, suggesting more of a chaperone-like identity than that of the classical nature of a receptor [14].
It is described that S1R agonists promote S1R dissociation from the endoplasmic reticulum binding immunoglobulin protein (BiP), resulting in S1R chaperone activity in the cells. In contrast, S1R antagonists reinforce the association, blocking the action of S1R agonists. This activity could be evaluated by the ability to modulate S1R–BiP dissociation, quantified by ELISA after immunoprecipitation [13]. Firstly, this well-validated cell-based assay was used to analyze compound 7i for its in vitro activity. The reference agonist PRE-084 (10 µM) significantly causes the dissociation of the S1R–BiP complex, while the antagonist NE-100 (10 µM) has no effect on S1R–BiP association (Figure 2). NE-100 also blocks the action of PRE-084, validating its agonist effect. Compound 7i (1 and 10 μM) significantly causes a dose-dependent dissociation of the S1R–BiP complex. Its effect was blocked by NE-100 (10 µM), demonstrating the agonist capacity of compound 7i. Dissociation was also observed after co-treatment with PRE-084 and compound 7i, showing that compound 7i does not block the action of PRE-084 which validates the absence of antagonist capacity.

In vitro compound 7i agonist activity evaluation.
The effect of compound 7i (1 and 10 µM) on S1R–BiP association (30-min incubation) was analyzed through co-immunoprecipitation with an S1R antibody (red). BiP level was measured using ELISA. PRE−084 (blue) and NE−100 (yellow) were used for agonist and antagonist of reference, respectively (10 µM). Agonist activity was validated by blockade by NE−100 (yellow hatching). The absence of antagonist activity was validated with PRE−084 (blue hatching). Data are presented as mean ± SEM of n = 6 mice per condition. *** p < 0.0001 vs. non treated group, ### p < 0.0001 vs. PRE-084 or 7i 10 μM group. Dunnett’s test.
Secondly, two well-described behavioral tests, validated for the evaluation of S1R activity in vivo, were performed. Compound 7i significantly attenuated dizocilpine-induced learning deficits at 0.5 mg/kg in the Y-maze test and at 0.1 and 0.5 mg/kg in the passive avoidance test (Figure 3). The beneficial effect of compound 7i in the two tests is prevented by treatment with the S1R antagonist NE-100 at 3 mg/kg, which is devoid of any effect by itself.

In vivo compound 7i agonist activity evaluation.
The effect of compound 7i (0.5 to 5 mg/kg, i.p., red) was analyzed with dizocilpine-induced learning deficit analysis (V, vehicle) through spontaneous alternation performances (panels (A,C)) and passive avoidance latency (panels (B,D)). Agonist activity was validated by NE-100 blockade (3 mg/kg, i.p., yellow). Data are presented as mean ± SEM of n = 6 mice per condition. * p < 0.05, ** p < 0.01 vs. (vehicle + vehicle)-treated group, # p < 0.05, ## p < 0.01 vs. (Dizocilpine + vehicle)-treated group, oo p < 0.01 vs. (Dizocilpine + 7i)-treated group. Dunn’s test.
These results confirm the S1R receptor agonist effect of compound 7i. Those in vivo behavioral tests also highlight the bi-phasic dose–response curve that is very regularly observed in the biological responses of S1R agonists [15]. The effects of compound 7i on behavior are visible at a concentration that ranges from 0.1 to 1 mg/kg.
2.3. In Vivo Pharmacology
2.3.1. Preliminary Pharmacokinetic Analysis
A drug candidate for CNS pathologies needs to penetrate and stay in the brain tissue for a sufficiently long time. One of the major challenges in CNS drug discovery and development is also to achieve the right balance between the free fractions in the blood and brain. In 2010, Wager et al. provided guidance for the design of successful CNS drug candidates based on several physicochemical drug properties [34]. The median optimal values were found to be partition coefficient (logP) = 2.8, molecular weight (MW) = 305.3, polar surface area (TPSA) = 44.8 Å2, hydrogen-bond donor (HBD) = 1 and pKa = 8.4. Compound 7i has MW of 316 Da; logP of 3.5; TPSA of 32 Å2; one HBD and possesses a basic amine with a pKa value of 9.1. Therefore, compound 7i displays desirable theoretical physicochemical properties predicting good CNS penetration. The blood–brain ratio remains a key analytical parameter that indicates the brain-targeting ability of neurotherapeutics with their brain bioavailability. It is for this purpose that the quantification of compound 7i was performed in plasma and brain homogenate samples. As the ultimate goal of pharmacological development is therapeutic establishment, oral (p.o.) administration was prioritized. The concentration time profiles were also carried out at 1 mg/kg after intravenous (i.v.) administration in order to define the pharmacokinetic parameters of compound 7i. In fact, the i.v. enteral route allows for relatively precise drug concentrations to be achieved in plasma since bioavailability is not a concern due to the device delivering the drug directly into the bloodstream. Conversely, in the oral parenteral route, blood draining in the gut passes through the liver, which is a major site of drug metabolism, before reaching the systemic circulation. The pharmacokinetic parameters obtained are listed in Table 7. The time needed to reach Cmax (tmax) was set to 0 after i.v. administration and was less than 30 min after p.o. administration (Figure S2). The maximal concentration (Cmax) in plasma after i.v. administration was determined as 180 ng/mL. Concentrations at 30 min (C30) after i.v. administration were more than 10 times higher than after p.o. administration, whether in the plasma or in the brain. After oral administration, compound 7i showed a distribution volume (Vd) higher than the total blood volume in mice (85 mL/kg), indicating extravascular distribution. Total clearance was found to be 3.8 mL/min after i.v. administration. This value was increased by 20 times when administration was performed orally. After i.v. administration, it takes 44.5 min to eliminate half of the circulating compound 7i (t1/2 el). Plasma t1/2 el was reduced to half 25.5 min after p.o. administration. After oral administration, the average time taken to arrive in the bloodstream (mean absorption time, MAT) was estimated as 16.5 min with 5% bioavailability. In the brain, clearance (CLbrain) was found to be 1.8 mL/min after i.v. administration. As observed in plasma, this value was increased by 20 times after p.o. administration. Brain elimination half-life (t1/2 el) was estimated as 96.4 min and 78.9 min after i.v. and p.o. administration, respectively. Diffusion in the brain was estimated as 62%. A [brain]/[plasma] ratio of 3 was observed regardless of the method of administration.
| i.v | p.o | |
|---|---|---|
| Plasma | ||
| tmax (min) | 0 | ≤30 |
| Cmax (ng/mL) * | 180 | NA |
| C30 (ng/mL) | 79.8 | 6.4 |
| AUC t∞/AUC ∞ | 0.9 | 6.1 |
| Vd (mL/kg) | 6.1 | 168.0 |
| CLT (mL/min) | 3.8 | 74.9 |
| t1/2 el (min) | 44.5 | 25.5 |
| MAT (min) | 0 | 16.5 |
| F (%) | 100 | 5 |
| Brain | ||
| tmax (min) | ≤30 | ≤30 |
| C30 (ng/mL) | 234.3 | 20.7 |
| AUC t∞/AUC ∞ | 5.9 | 7.6 |
| CLbrain (mL/min) | 1.8 | 12.8 |
| t1/2 el (min) | 96.4 | 78.9 |
| D (%) | 61.7 | NA |
| Brain/Plasma | 2.9 | 3.2 |
Pharmacokinetic parameters of compound 7i. Compound 7i was i.v. and p.o. administrated at 1 mg/kg. Analyses were performed in plasma and the brain. Maximal concentration (Cmax), time to reach Cmax (tmax) and concentration at 30 min (C30) were analyzed. The correct distribution of the time-points was validated using area under the curve (AUC) parameters (AUC t∞/AUC ∞). Distribution volume (Vd), total clearance (CLT), brain clearance (CLbrain), half-life of elimination (t1/2 el), mean absorption time (MAT), bioavailability (F) and diffusion in the brain (D) were calculated. The [brain]/[plasma] ratio was calculated at 30 min. Data were obtained using a median value of n = 3 mice per time-point. * for simulated, NA for not available.
Pharmacokinetic modeling was performed using the non-compartmental method (Figure 4). Differences in time were observed and are probably the result of a limited number of individuals.

Pharmacokinetic modeling using the non-compartmental method. Compound 7iwas administrated through two methods, i.v. (panel (A)) and p.o. (panel (B)), at 1 mg/kg.
2.3.2. Effects of Systemic Administration of the Selective 7i Drug on the Cardiovascular System
hERG is essential for normal electrical activity in the heart, and it has been known for a long time that arrhythmia can be induced by a blockade of this channel by a diverse group of drugs. This side effect is a common reason for drug failure in preclinical safety trials [35] because hERG channel inhibition can potentially lead to cardiac repolarization (i.e., QT interval). Electrocardiographic QT interval prolongation is the most widely used risk marker for ventricular arrhythmia potential and is thus an important component of drug cardiotoxicity assessments (Figure S3). As compound 7i inhibits hERG in vitro at micromolar range, the effect of compound 7i on ECG, and specifically QT interval duration was analyzed in vivo. The positive control quinidine dose-dependently prolonged the QT interval 6 to 10 min after intraperitoneal (i.p.) injection at 10 mg/kg and 100 mg/kg, while no alteration in cardiac repolarization was observed after administration of increasing concentrations (0.5 to 10 mg/kg) of compound 7i (Table 8). In addition, the QT interval also remained stable after longer acquisition times. Furthermore, compound 7i did not alter heart rate, nor PR or QRS duration. This present study allows us to conclude that compound 7i does not induce a pro-arrhythmic effect.
| QT interval | |||||||
|---|---|---|---|---|---|---|---|
| Time after Injection | |||||||
| Dose (mg/kg) | Basal | 3 min | 6 min | 10 min | 20 min | 30 min | |
| Vehicle | 51 ± 2 | 46 ± 3 | 45 ± 4 | 56 ± 9 | 62 ± 7 | 63 ± 11 | |
| 7i | 0.5 | 49 ± 3 | 48 ± 4 | 46 ± 4 | 53 ± 3 | 59 ± 4 | 58 ± 3 |
| 1.0 | 48 ± 1 | 45 ± 2 | 46 ± 2 | >49 ± 1 | > | ||
| >5.0 | >46 ± 3 | >43 ± 1 | >42 ± 2 | >43 ± 2 | > | ||
| 10.0 | 46 ± 1 | 43 ± 1 | 45 ± 3 | 49 ± 2 | 52 ± 1 | 54 ± 1 | |
| Quinidine | 10.0 | 46 ± 2 | 50 ± 2 | 64 ± 6 | 65 ± 5 * | ||
| 100.0 | 47 ± 4 | 58 ± 3 | 84 ± 7 * | 76 ± 4 * |
In vivo QT interval analysis. QT intervals measured via ECG under isoflurane anesthesia in mice injected with compound 7i, quinidine (positive control) or vehicle control. Data are presented as mean ± SEMof n = 2–3 mice per group. Differences between baseline QT intervals and QT intervals following i.p. injections were tested. * for p < 0.05 versus basal QT interval in the same group. Wilcoxon–Mann–Whitney U test.
2.4. In Vivo Efficacy of the Selective 7i Agonist in an MS Experimental Model
Experimental autoimmune encephalomyelitis (EAE) is a very important model for the exploration of new treatment options. Within the CNS, it reflects several pathomorphological features of MS, such as perivascular immune cell infiltration, activation of microglia and astrocytes, all contributing to demyelination and axonal loss. Relapsing EAE (R-EAE) was induced in female SJL/J mice using proteolipid protein peptide (PLP 139-151) [36,37], which classically peaked after 15 days and presented a maximal clinical score of 4.0 [27,38,39]. As compound 7i’s effects on behavioral tests are visible at a concentration range of 0.1 to 1 mg/kg, the treatment was administrated at 0.5 and 1 mg/kg in EAE mice. Daily injections were performed for 15 days when the animals reached a score 2 (i.e., tail atony and/or clumsy gait). EAE disease course was followed for 80 days in order to observe the second relapse. Daily scoring of clinical signs in a blinded manner showed that EAE—vehicle (control) mice experienced onset at day 16 (D16 ± 1) after immunization (Figure 5A,B) and presented a clinical score ≥ 2 for 8 days (first relapse). Animals kept a score slightly above 2 for 30 days before experiencing a less intense second relapse.

The curative effect of compound 7i in the multiple sclerosis (MS) experimental model.
Mice were immunized at day 0 (D0) and their clinical course was followed for 80 days (panels (A,C)). Compound 7i was administrated daily for 15 days when mice reached a score of 2 (panels (B,D)). Five different groups were analyzed depending on the route of administration (i.p. or p.o.) and concentration (0.5 and 1 mg/kg). The involvement of S1R in the beneficial effect of EAE was analyzed during the first relapse (panel B). S1R binding sites were blocked by i.p administration of BD-1047 (10 mg/kg) 20 min before compound 7iadministration. Data are presented as mean ± SEM of n = 5–6 mice per condition. ns, non-significant. **** p < 0.0001, ### p < 0.001 vs. EAE—vehicle group. * or # p < 0.05 vs. EAE—vehicle group; o p < 0.05 vs. EAE—BD-1047 and 7i group. Wilcoxon–Mann–Whitney U test.
Initially, compound 7i was classically administrated intraperitoneally in a curative protocol. Compound 7i’s effect was visible from the first days of treatment, with a more pronounced effect at a concentration of 1 mg/kg (Figure 5B). No second relapse was observed, regardless of the concentration tested (Figure 5A). When EAE disease course was followed for a longer time, EAE global evolution was significantly reduced when animals received the compound at 0.5 mg/kg. To confirm that the biological activity of compound 7i required the presence of S1R, S1R binding sites were blocked with the S1R antagonist BD-1047, given intraperitoneally (10 mg/kg) 20 min before compound 7iadministration [27,40]. A similar clinical evolution was observed with the vehicle and BD-1047 pretreatment (Figure 5B). The same significant difference was observed when the EAE—7i group was compared to the EAE—vehicle and EAE—BD-1047 and 7i group, showing that BD1047 blocked the effects of compound 7i on EAE development, thus validating S1R specific mediated action. BD-1047 alone displayed no significant effect on EAE development [27].
In a second step, compound 7i was administrated orally in the same curative protocol scheme. Very interestingly, a significant decrease in EAE course was observed after administration at 0.5 mg/kg (Figure 5C). This significant decrease in clinical intensity was observed as soon as the treatment was implemented (Figure 5D). Compound 7i’s effect was maintained during the 15 days of administration, where animals reached grade 1. It is also important to note that orally treated animals do not exceed grade 2 during the entire disease course. No rebound effect was observed, as in i.p. administration (Figure 5C).
3. Discussion
The drug development process is typically divided into four major steps: discovery, proof of concept, preclinical development and clinical trial. In this paper, compound 7i was used, whose synthesis and pharmacological evaluation has previously been investigated [28]. The implementation of a typical preclinical program demonstrates that, in addition to its excellent affinity for S1R, its high selective profile and its absence of cytotoxicity, compound 7i has all the characteristics of an excellent drug candidate specifically targeting S1R. Its application in CNS disease, specifically in multiple sclerosis (MS), was demonstrated.
Knowledge of the possible drug metabolites that can be generated, as well as quantification and analysis of their individual activity, is important as it can potentially impede the future translation of therapeutic compounds into the clinic. To find out if there is an interspecies difference in metabolic stability profile, compound 7i analysis was performed in mouse and human liver microsomes. The most important metabolite was identified as the demethylated compound, named compound 7i-deMe. Compound 7i-deMe also retains a good affinity for S1R with a high selectivity index. Preliminary ADME demonstrate that compounds 7i and 7i-deMe present solubility, permeability and plasma characteristics compatible with pharmacological development and that the route of administration of those compounds should be non-intravenous. Compounds have no impact on CYP inhibition in vitro, except CYP2D6. It was then decided to evaluate the potential of compound 7i for drug–drug interaction (DDI). This step was taken early on in the study because, in the case of chronic neurodegenerative diseases, patients are likely to be prescribed medications to manage blood pressure, cardiovascular disease, joint inflammation, diabetes and other conditions associated with aging. Identifying the specific transporter for which a new compound is an inhibitor or a substrate improves drug bioavailability and efficacy, but also helps prevent adverse effects. Particular interest has been shown to BCRP and P-gp, expressed at the BBB. No drug transporter inhibition was observed, except that OCT1 and OCT2 were inhibited by compound 7i-deMe. However, compound 7i conversion into compound 7i-deMe allows it to maintain part of its biological activity. As P-gp is crucial for absorption through active transport and CYP induction can affect drug efficacy through reducing plasma half-life, or drug toxicity if elevated levels of toxic metabolites are formed, assessment of those parameters was added to the analysis. The results obtained demonstrate that compound 7i has all the necessary features to continue preclinical program development. Safety pharmacology and toxicology studies have also been undertaken. In fact, the potassium channel hERG is essential for normal electrical activity in the heart, and arrhythmia can be induced by a blockage of hERG by a surprisingly diverse group of drugs [35]. Given the similarity of reported S1R pharmacophore models to those postulated for hERG [41], and the direct interaction between S1R and hERG [42], compound 7i was shown to inhibit hERG in vitro with an IC50 of 1 µM. As hERG channel blockers can cause long QT syndrome, which results in an increased risk of patients developing “torsades de pointes,” a fatal ventricular arrhythmia, the effect of systemic administration of compound 7i on ECG interval duration, and specifically QT interval duration, was analyzed. It has been demonstrated that compound 7i does not alter heart rate, nor PR or QRS duration in vivo. No prolongation of the QT interval was observed, leading to the conclusion that compound 7idoes not induce any pro-arrhythmic effect and confirming that development can be continued.
To complete this first phase of evaluation, cell cytotoxicity was classically analyzed by cell number counting. S1R is located at the MAM and is implicated in Ca2+ signaling between ER and mitochondria [13,14]. After activation, S1R also modulates several receptors, ion channels, transporters and enzymes localized at the plasma membrane [17,18]. No impact from compound 7i was observed on intracellular free calcium, nuclear size, membrane permeability or mitochondrial membrane potential, certifying the absence of cytotoxic adverse effects. Furthermore, standard procedures established to analyze genetic toxicology for small molecules were used. Ames tests and induced chromosome damage (micronucleus assays) were performed. Finally, attention was focused on immunotoxicology that can manifest in a variety of ways, with one of the most prominent effects being immunomodulation and immunosuppression. Immunosuppressive agents cause a significant alteration in the body’s immune system, which enables opportunistic infections and malignancies [43]. Immunosuppressants decrease immunosurveillance, causing a subsequent lack of ability to fight infections. In MS, increased patient vigilance has been encouraged after several incidents of progressive multifocal leukoencephalopathy (PML) were reported in Natalizumab patients. Conversely, some drugs, specifically those used in cancer therapy, can promote the activation and expansion of the immune system and induce a wide range of immune-related adverse effects [44]. The S1R agonist 1(S), containing the tetra-hydroisoquinoline-hydantoin structure, was shown to decrease the magnitude of inflammation. The effect was associated with an increase in the proportion of B-cell subsets and regulatory T cells in the spleen and cervical lymph nodes [27]. Very interestingly, compound 7i has no depleting or enhancing effect on several human immune cell types, including B, T, Th17 and regulatory T lymphocytes. Activated CD4, activated CD8 and natural regulatory T cells also retain their sensitive capacities and their ability to proliferate after stimulation, which confirms the safety of compound 7i.
CNS-targeted diseases are unanimously considered to be one of the most important and difficult therapeutic challenges of our time. Accumulating evidence highlights the preclinical efficacy of selective drugs targeting S1R that act as agonists. Compounds showing S1R binding could also be allosteric modulators that have no pharmacological activity by themselves. Establishing the characterization of the activity of compound 7i was crucial at this stage of the development phase. Evaluation of its capacity was firstly analyzed in vitro regarding S1R–BiP dissociation. The activity was then confirmed in vivo via the use of validated behavioral tests. Therefore, we were able to confirm that function of compound 7i as a pharmacological agonist able to elicit a physiological response. The use of concentrations ranging from 0.1 to 5 mg/kg made it possible to highlight the bi-phasic bell-shaped dose–response curve, a common feature of S1R agonists [15] which was originally introduced through the notion of hormesis, defined as paradoxically beneficial effects seen with low doses and less beneficial effects at higher doses [45]. Compound 7i’s maximum effects were visible at 0.5 to 1 mg/kg. It is important to note that S1R oligomerization states may also be important in regulating its functions [46]. S1R exists as dimers, tetramers, hexamers, octamers and perhaps even higher order oligomers. Oligomeric states of S1R could be stabilized by ligands [47]. The monomer form has been shown to bind to protein partners at the plasma membrane as a functional unit [48]. These data further enabled us to begin our in vivo preclinical studies. As the ultimate goal of pharmacological development is therapeutic establishment, pharmacokinetics (PK) parameters were evaluated for p.o. administration. Diffusion in the brain and the [brain]/[plasma] ratio demonstrated that compound 7i exhibits excellent features for CNS diseases at nanomolar concentration. A comparison with the data from PRE-084, the prototypical drug used to uncover the potential therapeutic benefits of S1R [49], enables us to highlight that compound 7i has all the qualities necessary to serve as a promising S1R-targeted drug [50].
Current MS treatments demonstrate high success in limiting new relapses and the accumulation of focal lesions; however, reparative approaches are still needed. Remyelinating treatment, as well as neuroprotective strategies, may lead to the reversal of neurological deficits. It is pivotal to highlight that development of oral drugs with sufficient bioavailability remains the forefront of preclinical research. EAE is an induced inflammatory disease of the CNS which follows the induction of immune response against CNS-specific antigens. A number of different models have been developed that can mimic certain stages of the MS disease; these commonly used models have directly contributed towards the development of a number of first-line MS treatments [37,51]. Compound 7i was tested in a curative approach, for 15 days, in PLP-induced disease in SJL/J mice. This experimental chronic model offers a unique and interesting relapsing remitting course (R-EAE) comparable to the most common form of MS. Spinal cord demyelination and axonal damage are pathological markers of this model [37]. Treatment was therefore implemented during clinical disease, when animals reached clinical grade 2 (i.e., tail atony and/or clumsy gait). The compound was administrated at 0.5 and 1 mg/kg to induce an optimal effect, based on behavioral tests showing agonist activity and previous data from the laboratory [27]. EAE disease course was followed for 80 days in order to observe the emblematic acute (first relapse) and chronic (second relapse) phases of this R-EAE model. Decreases in EAE course were observed when compound 7i was classically administrated intraperitoneally. As in behavioral tests, a bi-phasic bell-shaped dose–response curve was observed, with a greater significant effect on EAE global evolution at the lowest concentration. No second relapse was observed regardless of the concentration tested. The involvement of S1R was validated by using BD-1047 to saturate S1R binding sites before compound 7i administration. Very interestingly, a significant decrease in EAE clinical intensity was also observed after oral administration of compound 7i at 0.5 mg/kg, with a more pronounced effect as soon as the treatment was implemented (first relapse). Disease progression was stopped at clinical grade 2 and the action of compound 7i was maintained at that time. Despite the interruption of the treatment, no rebound effect was observed.
As S1R controls ER stress and inflammatory processes in the cells, S1R agonists have been tested for a long time in preclinical studies and clinical trials for neuroprotection in neurodegenerative diseases, characterized by the progressive dysfunction of the structure and function of neuronal and glial cells and their network in the CNS. Many drugs interact with S1R and behave as agonists but are not selective. In the last few decades, S1R has emerged as a promising new target in MS. Eliprodil, an NMDA receptor antagonist with S1R affinity, has been shown to promote myelination in neuron–oligodendrocytes cocultures [52]. Dextrometorphan, an NMDA receptor antagonist and S1R agonist, demonstrated protective effects at low doses in EAE [53]. Additionally to dextometorphan, the mixed S1R and muscarinic receptor agonist ANAVEX2-73 (Blarcamesine), can also provide protection for oligodendrocytes and oligodendrocytes precursors and promote reparative therapy in MS [54]. Interestingly, the selective S1R agonist RC-33 demonstrated neurite elongation promotion in a rat dorsal root ganglia experimental cellular model, supporting the potential of S1R agonists in MS treatment [24]. Finally, our laboratory demonstrated that a single i.p. injection of the S1R agonist 1(S), containing the tetrahydroisoquinoline-hydantoin structure, decreased the clinical progression of EAE disease and prevented mononuclear cell accumulation and demyelination in the brain and spinal cord [27]. Nevertheless, compound 1(S) has only moderate metabolic stability. In this study, we thus decided to use compound 7i, a simple benzamide-derived compound with excellent nanomolar affinity for S1R, selectivity for S2R, no cytotoxicity and good metabolic stability. It is important to note that extensive pharmacological profiling against 40 receptors relevant to CNS disease showed that it has a highly selective profile [28]. We have demonstrated that compound 7ipresents ADME properties, safety pharmacology and toxicology characteristics, as well as agonist capacity that makes it an excellent drug candidate for targeting CNS diseases. In an experimental model of MS, compound 7i was successfully administrated orally in a curative procedure. Bell-shaped response, a common feature of S1R agonists, was observed during behavioral tests as well as in EAE, with an optimum effect at the lowest dose. At the molecular level, S1R can indeed be found in monomeric and oligomeric forms in physiological conditions, and it has been shown that agonists favor monomers and/or dimers while antagonists favor tetramers, hexamers, octamers and perhaps even higher order oligomers [17,48]. Dimer and monomer forms may represent the functional active forms, while the oligomers of S1R may serve as a reservoir for the active forms. At low doses, agonists activate S1R by binding to monomers and dimers who will then directly interact with other partner proteins, carrying out S1R chaperone activity. The oligomer reservoir allows the amount of active forms to be kept constant. At higher doses, agonists also interact with tetramers/hexamers/octamers, thus favoring an antagonist state that will lead to a decrease in global S1R response [15]. It should also be hypothesized that the bi-phasic dose-dependent effect observed could have an impact at the cellular dynamics level. It could indeed be that a low dose of the agonist facilitates S1R action on MAM, whereas a high dose of the agonist impacts the action of S1R on more distant cell compartments [15].
Altogether, these findings confirm that S1R represents a promising target for the development of affordable drugs against MS. S1R agonists may also be useful for adjunct treatment of MS and/or further development in progressive MS due to their manifold properties. The results obtained with compound 7i confirm the positive role of S1R-selective benzamide-derived agonist compounds in the modulation of MS. This new drug candidate presents a moderate risk regarding its use in clinical trials. However, special attention should be paid to defining the optimum therapeutic window and the best concentration for use since S1R dose–response activity is bell-shaped for all drugs and blocked at high doses [15]. During clinical development, it will be important to keep in mind that lower doses have to be investigated as a priority. This most effective dose can only be found by relying on effective biomarkers [15,45]. It should also be interesting to analyze compound 7i’s mode of action (MOA) given that boosting S1R activity leads to intracellular calcium homeostasis restoration, the stabilization of mitochondrial physiology and cellular adaptive response facilitation [16]. More precisely, calcium dynamics, as well as oxidative stress, should be studied under pathological conditions, particularly in oligodendrocytes and neurons. Neuroinflammation should also be studied since immune cell and cytokine impact are localized in the CNS. In the broader context of other CNS-targeted diseases, the impact of S1R modulation by compound 7i on unfolded protein response, as well as on autophagy, could also be analyzed.

