Fragile-X Syndrome Is Associated With NMDA Receptor Hypofunction and Reduced Dendritic Complexity in Mature Dentate Granule Cells
By Suk-Yu Yau, Luis Bettio, Jason Chiu, ChristineChiu, and Brian R. Christie
TITLE=Fragile-X Syndrome Is Associated With NMDA Receptor Hypofunction and Reduced Dendritic Complexity in Mature Dentate Granule Cells
Excerpt from the article published in Frontiers in Molecular Neuroscience, volume 11, 17 January 2019 Doi: https://doi.org/10.3389/fnmol.2018.00495
Editor’s Highlights
- N-methyl-D-aspartate receptors (NMDAR) hypofunction in the hippocampal dentate gyrus contributes to cognitive impairments in Fragile X syndrome (FXS).
- The reduction in NMDAR function is associated with developmental deficits in dendritic arborization of neurons in the hippocampal dentate granule cell layer (GCL)
- Neurons with multiple primary dendrites that reside in the outer GCL in FXS display significantly smaller NMDAR excitatory post-synaptic currents (EPSCs).
- The effects of FMRP on NMDAR function and dendritic complexity may occur over time.
- A loss of FMRP causes significant deficits in both NMDAR function and dendritic arborization in mature neurons, and this could contribute to the aberrant neurogenic process and reduced cognitive performance that has been observed with the loss of FMRP.
Abstract
Fragile X syndrome (FXS) is the most common form of inherited intellectual disability. It is caused by the overexpansion of cytosine-guanine-guanine (CGG) trinucleotide in Fmr1 gene, resulting in complete loss of the fragile X mental retardation protein (FMRP). Previous studies using Fmr1knockout (Fmr1 KO) mice have suggested that a N-methyl-D-aspartate receptors (NMDAR) hypofunction in the hippocampal dentate gyrus may partly contribute to cognitive impairments in FXS. Since activation of NMDAR plays an important role in dendritic arborization during neuronal development, we examined whether deficits in NMDAR function are associated with alterations in dendritic complexity in the hippocampal dentate region. The dentate granule cell layer (GCL) presents active postnatal neurogenesis, and consists of a heterogenous neuronal population with gradient ages from the superficial to its deep layer. Here, we show that neurons with multiple primary dendrites that reside in the outer GCL of Fmr1 KO mice display significantly smaller NMDAR excitatory post-synaptic currents (EPSCs) and a higher α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) to NMDA ratio in comparison to their wild-type counterparts. These deficits were associated with a significant decrease in dendritic complexity, with both dendritic length and number of intersections being significantly reduced. In contrast, although neurons with a single primary dendrite resided in the inner GCL of Fmr1 KO mice had a trend toward a reduction in NMDAR EPSCs and a higher AMPA/NMDA ratio, no alterations were found in dendritic complexity at this developmental stage. Our data indicate that the loss of FMRP causes NMDAR deficits and reduced dendritic complexity in granule neurons with multiple primary dendrites which are thought to be more mature in the GCL.
Introduction
Cognitive impairment is a frequently reported issue for patients with fragile X syndrome (FXS), which is the most common form of inherited intellectual disability, and the leading single gene cause of autism spectrum disorder (Rousseau et al., 1991; Kabakus et al., 2006; Alanay et al., 2007). This syndrome is caused by the transcriptional silencing of the fragile X mental retardation 1 (Fmr1) gene, resulting in the loss or mutation of its product, fragile X mental retardation protein (FMRP), a RNA-binding protein that associates with polyribosomes and regulates translation (Brown et al., 2001; Zalfa et al., 2003). FMRP has been shown to repress the translation of several targets, including proteins critical for synaptic function (Zalfa et al., 2003; Darnell et al., 2011). Of particular interest for the current study is the identification of interaction partners that play roles in signaling pathways related to synaptic plasticity (Tcherkezian et al., 2010) and dendritic structure (Schenck et al., 2003; De Rubeis et al., 2013).
Using Fmr1 knockout (Fmr1 KO) mice, we and others have demonstrated that the cognitive impairments in FXS may be linked to a disruption in N-methyl-D-aspartate receptor (NMDAR)-dependent synaptic plasticity in the hippocampal dentate gyrus (DG) (Yun and Trommer, 2011; Eadie et al., 2012; Franklin et al., 2014; Bostrom et al., 2015, 2016). The NMDA receptor forms a heterotetramer between two obligatory GluN1 subunits and two GluN2 subunits. The GluN2 subunits are differentially expressed during development, with GluN2B subunits initially being more highly expressed than GluN2A subunits early in neuronal development (Wenzel et al., 1997). The impact of NMDAR on dendritic structure can also have functional implications, as neuronal models indicate that dendritic morphology can have a significant impact on neuronal firing patterns (Mainen and Sejnowski, 1996).
Activation of NMDARs is known to play an important role in dendritic arborization and spine morphogenesis during neuronal development (Tolias et al., 2005). NMDARs appear to contribute to spine and dendrite formation; however their exact role remains controversial. Using specific GluN2 antagonists in cultured cells, GluN2A subunits have been associated with dendritic arborization, while GluN2B subunits were associated with dendritic spine formation (Henle et al., 2012). In contrast, introducing the GluN2B subunits into ventral spinal neurons in culture enhanced dendritic arborization, an effect not observed with GluN2A subunits (Sepulveda et al., 2010). Recently we have shown that genetic deletion of the Glun2A subunit significantly decreases dendritic growth in maturing dentate granule cells (Kannangara et al., 2014), suggesting that NMDA hypofunction in the DG may affect dendritic arborization in this brain region that exhibits developmental regulated changes in neurogenic activity (Gil-Mohapel et al., 2013).
In the current study we sought to determine if the reduction in NMDAR function observed in Fmr1 KO animals was associated with developmental deficits in dendritic arborization of neurons in the hippocampal dentate granule cell layer (GCL) (Cameron and Mckay, 2001). The adult-born neurons of the DG have been shown to express primarily GluN2B subunits early on (Spampanato et al., 2012), but are also known to undergo extensive dendritic arborization as they migrate into an already extensively populated GCL (van Praag et al., 2002). Indeed, dendritic arborization and cell body positioning have been used to identify young and old neurons in the DG (Wang et al., 2000; Eadie et al., 2005). Newly generated neurons tend to be preferentially located in the inner layer of the GCL (Overstreet et al., 2004; Espósito et al., 2005; Redila and Christie, 2006), whereas more mature granule cells appear to be located in the outer GCL (Wang et al., 2000; Overstreet et al., 2004; Redila and Christie, 2006). Combined morphological and electrophysiological analyses also indicate that neurons in the outer GCL are morphologically more complex and thus have a lower series resistance than neurons in the inner GCL (Wang et al., 2000; van Praag et al., 2002; Kannangara et al., 2014). Using the location of neurons in the GCL as a means to select neurons for whole-cell patch clamp analyses, we investigated how the loss of FMRP affects NMDAR function and dendritic arborization of both younger and more mature hippocampal DG neurons.
…
Results
Morphological Difference of Biocytin-Filled Granule Cells Located in the Inner and Outer Granule Cell Layers
The neurons co-labeled with the immature neuronal marker doublecortin (DCX) displayed as the ones with a single primary dendrite were located in the inner cell layer (Figures 1A–C), while neurons co-labeled with the mature neuronal marker NeuN displayed multiple dendrites and were located in the outer cell layer (Figures 1D–F). Representative images showing differences in morphology and location of granule neurons in the GCL are demonstrated in Figures 1G–I.
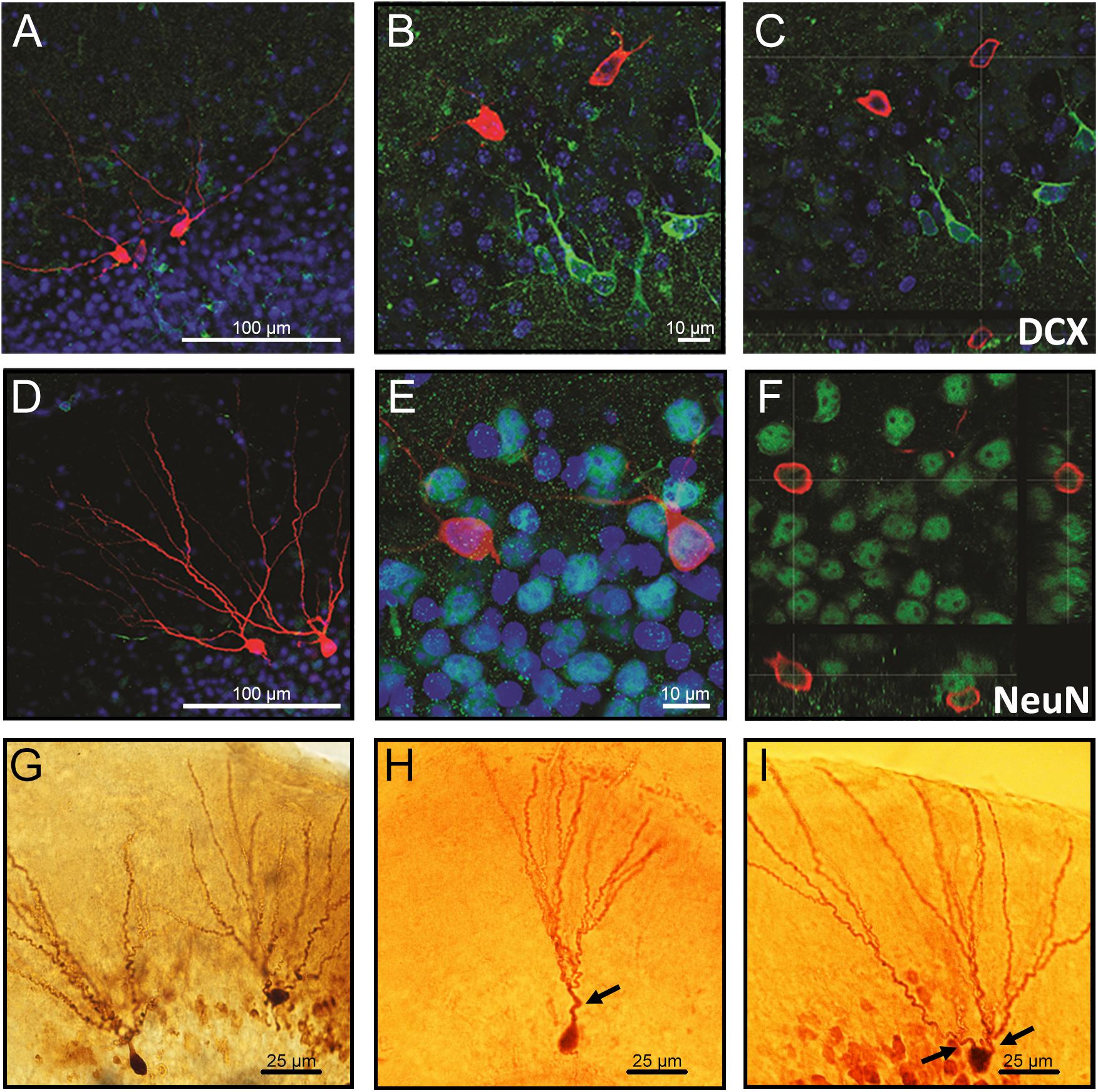
Confocal images of biocytin-filled cells in the granule cell layer (GCL) of the hippocampal dentate gyrus. (A–C) Biocytin-filled cells presenting multiple dendrites located in the outer GCL, while doublecortin-positive (DCX; immature neurons) cells located in the inner GCL layer. (D–F) Co-labeling of granule neurons projecting multiple dendrites from the soma with NeuN confirms that these cells are mature neurons. (G) Representative images of biocytin-filled neurons in the GCL of the hippocampal DG showing (H) an immature neuron localized in the inner layer and (I) a mature neuron localized in the outer cell layer. Red: biocytin-filled cells; Green: DCX (A–C) and NeuN (D–F); Blue: Dapi nucleus staining; Arrows: primary dendrites. Slice thickness: 350 μm.
Action Potential and Membrane Properties of Dentate Granule Cells in Fmr1KO Mice
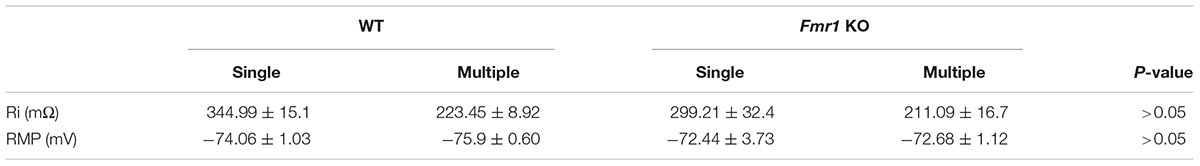
Figure 2A shows a representation of action potential trains in neurons with a single primary dendrite or multiple primary dendrites from WT mice. There were no significant differences in action potential frequency when comparing granule cells from WT to Fmr1 KO animals (single primary dendrite: F1,20 = 0.007, P = 0.933, Figure 2B; multiple primary dendrites: F1,24 = 1.158, P = 0.293, Figure 2C). However, granule neurons with multiple primary dendrites from Fmr1 KO mice displayed a trend toward a higher maximum frequency of action potential (Student’s t-test, WT: P = 0.156; Fmr1 KO: P = 0.05, Figure 2D), and a trend toward a lower input resistance when compared with the ones with a single primary dendrite (Table 1).
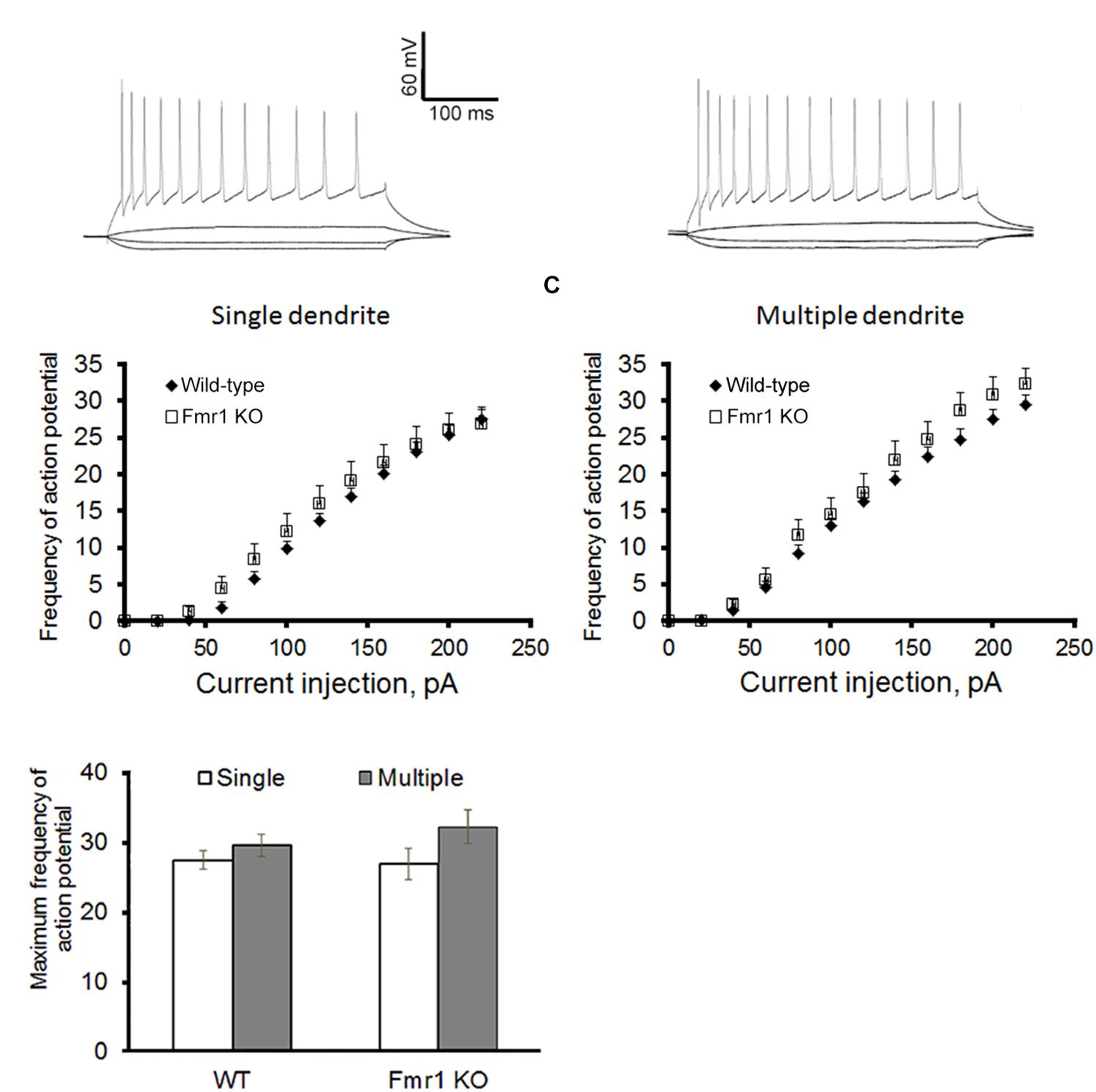
Action potential firing pattern of granule cells. (A) Representative traces of action potential train in neurons with single primary dendrite (left) or multiple primary dendrites (right) from WT mice. (B) Loss of FMRP did not affect firing pattern of granule neurons with single primary dendrite or (C) multiple primary dendrites. (D) However, neurons with multiple primary dendrites display a trend toward a higher maximum action potential firing rate when compared to the ones with a single primary dendrite. Neurons with single primary dendrite: WT: n = 12, Fmr1 KO: n = 8; neurons with multiple primary dendrites: WT: n = 16, Fmr1 KO: n = 8 (five mice per group).
Decreased NMDA EPSCs in Hippocampal Granule Cells From Fmr1 KO Mice
For all recordings, AMPAR and NMDAR EPSCs were evoked using increasing stimulation intensity to construct I/O curves and determine the maximum response size. Representative traces acquired from granule neurons are depicted in Figure 3A. Similar levels of AMPAR-mediated EPSCs were recorded in younger granule cells from both experimental groups (F1,25 = 1.36, P = 0.254, Figure 3B), while a trend toward a reduction in NMDAR response size was observed in the Fmr1 KO mice when compared to their WT littermates (F1,25 = 3.69, P = 0.067, Figure 3C). No alterations were found in AMPAR-mediated EPSCs of mature cells (F1,20 = 1.82, P = 0.194, Figure 3D), but a significant reduction in NMDAR-mediated EPSCs was observed in mature granule cells from Fmr1 KO mice (F1,20 = 14.843, P = 0.001, Figure 3E).
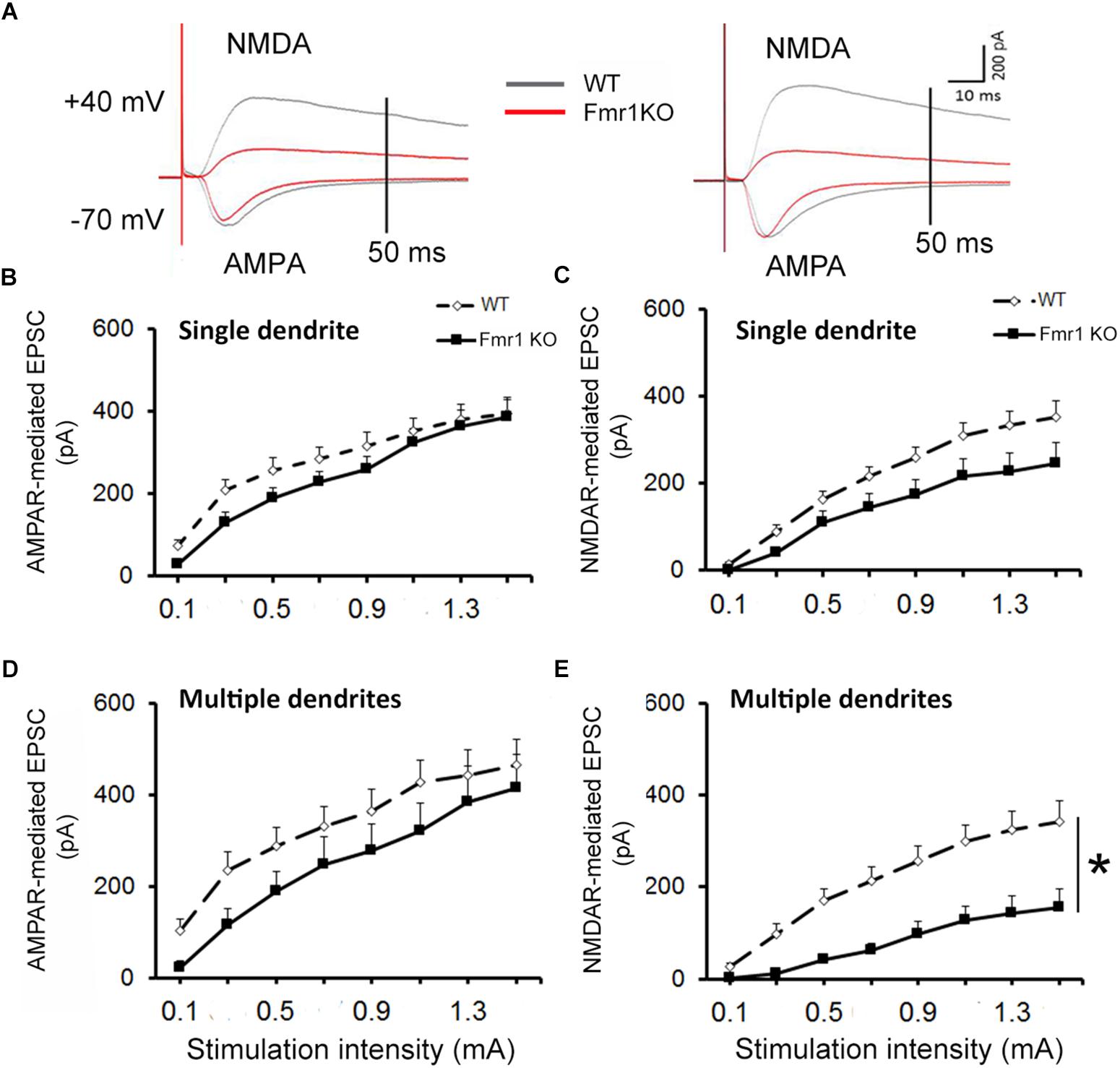
Decreased NMDAR-mediated EPSC in dentate granule cells of Fmr1 KO mice. (A) Representative traces of granule neurons. Neurons with a single primary dendrite (left panel) and neurons with multiple dendrites (right panel) at maximal amplitude were shown. (B) No significant difference in AMPAR-mediated EPSC was observed between Fmr1 KO and WT mice. (C) A trend toward a lower NMDAR-mediated EPSC was found in Fmr1 KO mice when compared to WT mice (P = 0.067). (D) No significant difference in AMPAR-mediated EPSCs of older granule neurons was observed between Fmr1 KO and WT mice; (E) but a significant decrease in NMDAR-mediated EPSC was observed in Fmr1 KO mice when compared to WT mice. ∗P < 0.005 (n = 9–14; five mice per group; ANOVA, repeated measures).
The analyses of the maximum amplitude of AMPAR-mediated EPSCs in neurons with a single primary dendrite did not reveal a significant difference between genotypes (P = 0.89), but a trend toward a reduction in NMDAR EPSCs was observed in cells from Fmr1 KO mice in comparison with WT mice (P = 0.09, Figure 4A). This trend was also observed in the AMPAR/NMDAR ratio (P = 0.07, Figure 4B). A significant decrease in absolute maximum NMDAR-mediated EPSCs was found in mature neurons from Fmr1 KO mice (P < 0.01, Figure 4C), as well as an increase in the AMPAR/NMDAR ratio (P < 0.05, Figure 4D) when compared to their WT counterparts. The amplitude of individual AMPAR- and NMDAR-mediated responses from hippocampal neurons at distinct developmental stages is depicted in Figure 4E.
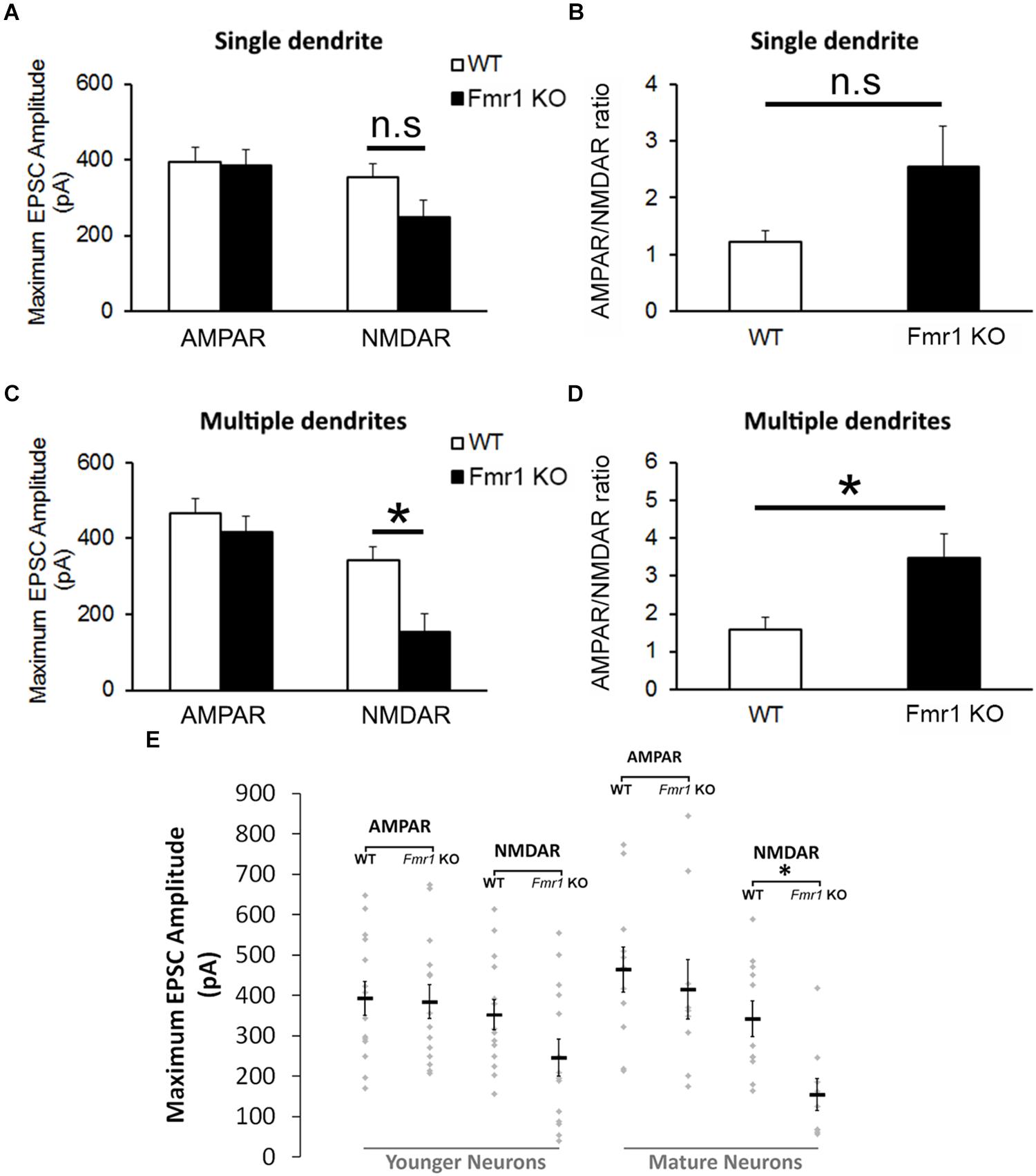
Altered NMDA currents and AMPA/NMDA ratio in Fmr1 KO mice. (A) No difference was found in AMPAR-mediated EPSCs from neurons with a single primary dendrite, but a trend toward a reduction in NMDAR-mediated EPSCs (P = 0.09) and (B) an increase in AMPAR/NMDAR ratio (P = 0.07) were observed in Fmr1 KO mice. (C) Although neurons with multiple primary dendrites from both groups demonstrated similar responses of AMPAR-mediated EPSCs, a significant decrease in NMDAR-mediated EPSCs (D) and a significant higher AMPAR/NMDAR ratio were found in Fmr1 KO mice when compared to WT mice. (E) Scatterplot showing the amplitude of AMPAR- and NMDAR-mediated responses of individual neurons at distinct developmental stages in the hippocampal dentate gyrus of WT and Fmr1 KO mice. ∗P < 0.05; n.s., non-significant (n = 9–14; five mice per group; Student’s t-test).
Fmr1 KO Mice Present Decreased Dendritic Complexity in More Mature Granule Cells, but Not Younger Granule Cells
Examples of biocytin filled cells used for the analysis of dendritic complexity are shown in Figures 5A,B. Sholl analysis revealed that WT and Fmr1 KO mice presented similar dendritic measures (dendritic length: F1,18 =0.185, P = 0.673, Figure 5C; dendritic branching: F1,18 = 0.473, P = 0.501, Figure 5D). Conversely, cells with multiple primary dendrites from Fmr1 KO mice displayed a drastic reduction in both dendritic length (F1,22 =26.291, P < 0.005, Figure 5E) and number of intersections (F1,22 = 15.301, P = 0.001, Figure 5F) when compared to their WT littermates. These findings indicate that the loss of FMRP may lead to a significant decrease in the development of dendritic complexity in more mature neurons.
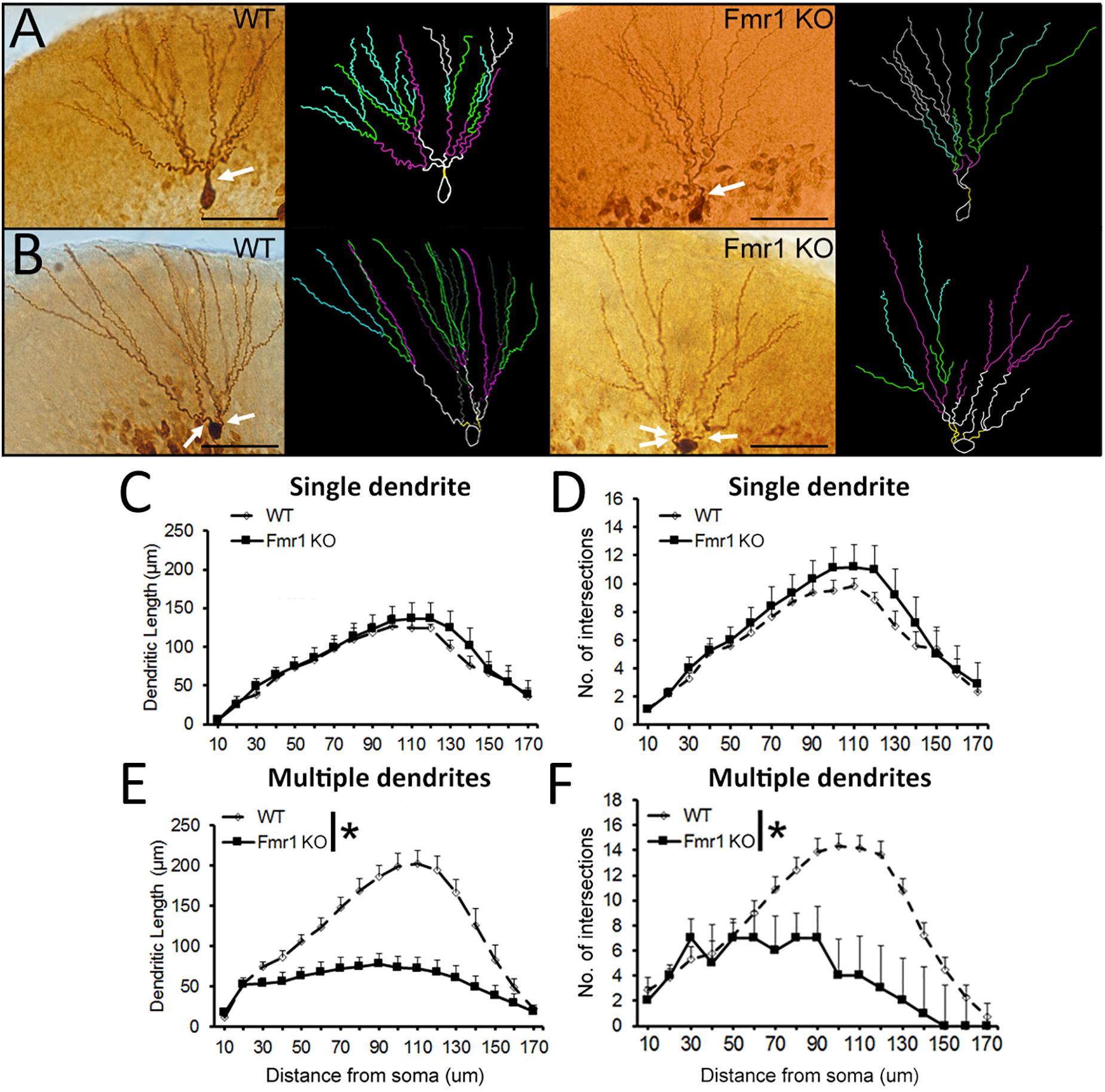
Sholl analysis of biocytin-filled granule cells. (A) Representative images of younger and (B)mature granule neurons from WT and Fmr1 KO mice. Scale bar: 50 μm. (C,D) There were no differences in dendritic length and number of intersections in younger granule cells between WT (n = 10) and Fmr1 KO mice (n = 8). (E,F) Dendritic length and number of intersections in more mature granule cells were significantly decreased in Fmr1 KO mice (n = 10) when compared to WT mice (n = 12). Arrows: primary dendrites (yellow traces). ∗P < 0.005 (five animals per group; ANOVA, repeated measures).
Discussion
The current study indicates that a loss of FMRP results in a significant reduction in NMDAR-mediated EPSCs and a concomitant decrease in dendritic complexity in hippocampal granule cells. Our findings also indicate that these deficits are primarily observed in the mature granule neurons which are with multiple primary dendritic processes arising from the soma, and are primarily located in the outer GCL. On the other hand, younger granule neurons that reside in the inner GCL, did not show a significant change in dendritic complexity, although they did show a trend toward a reduction in NMDAR EPSCs induced by the lack of FMRP. These data further support our previous findings indicating that Fmr1 KO mice present a dysfunction in NMDAR in the hippocampal DG (Bostrom et al., 2015; Yau et al., 2016a), and show for the first time that these deficits are preferentially associated with a reduction in dendritic arborization in a specific population of granule cells in the outer GCL.
Dendritic morphology can affect synaptic plasticity by modulating neuronal firing rates, as well as altering the propagation of EPSPs and action potentials (Mainen and Sejnowski, 1996; Magee, 2000; Vetter et al., 2001). Loss of FMRP has been shown to reduce pruning of dendrites in the somatosensory cortex (Galvez et al., 2003) and in mitral cells of the olfactory bulb (Galvez et al., 2005), suggesting that this protein plays an important role in the development of dendritic processes. In the current study, we found that the loss of FMRP leads to a significant decrease in dendritic complexity in a specific subpopulation of granule neurons located in the outer GCL. This population of newborn neurons present multiple primary dendrites arising from the cell body and has been previously classified as being “mature neurons” based on morphological and electrophysiological analyses (Liu et al., 1996; Wang et al., 2000; Bartesaghi and Serrai, 2001; Kannangara et al., 2014). Our findings are in line with previous studies showing that the lack of FMRP leads to significant impairments in neurite extension of primary neural progenitor cells derived from the hippocampal DG (Guo et al., 2011). In addition, an in vivostudy using retroviral labeling with green fluorescent protein found that mature newborn neurons from Fmr1KO mice present a decrease in dendritic complexity when compared to their WT counterparts (Guo et al., 2012). Our study corroborate these findings and provide further evidence indicating that the lack of FMRP affects hippocampal newborn cells in an age-dependent manner.
The regulation of neuronal morphology and synaptic modifications depends on the remodeling of the actin cytoskeleton, which in turn has been previously shown to be regulated by FMRP (Dictenberg et al., 2008; Nolze et al., 2013). Thus, it is possible that the deficits in dendritic length and branching observed in the present study are related to alterations in the organization of actin filaments induced by the lack of FMRP in the hippocampal DG. These changes may be related to the NMDAR hypofunction we observed, since these receptors are known to play a critical role in activity-dependent development of dendritic arbors and synaptogenesis (Nikonenko et al., 2002; Wong and Ghosh, 2002). It may be that the deficits in NMDAR function observed in our study in turn affect several NMDAR-dependent proteins who exert an influence on dendritic morphology. These may include extracellular signal-regulated kinases (Vaillant et al., 2002), Ca2+/calmodulin-dependent protein kinase II (Shi and Ethell, 2006), Rho family GTPases (Tolias et al., 2005) and glycogen synthase kinase 3 β (GSK-3β) (Rui et al., 2013). These proteins are interesting targets for future studies, particularly GSK-3β, since alterations in the translation of this protein seem to play a major role in the impaired differentiation of hippocampal neurons in FXS (Luo et al., 2010; Guo et al., 2012).
Prior research indicates that GluN2A subunit-containing NMDARs are important for dendritic arborization (Kannangara et al., 2014), while GluN2B may be more critical in regulating spine formation (Henle et al., 2012). Interestingly, we have previously shown that NMDAR hypofunction is associated with a significant decrease in the expression of both GluN2A and GluN2B subunits in the DG of Fmr1 KO mice (Bostrom et al., 2015). However, because FMRP is a translational repressor, it is still not clear how its deletion leads to a reduction in the expression of NMDAR subunits, as well as to what extent it affects temporal changes in GluN2A and GluN2B expression in younger and more mature dentate granule cells. One factor that could be contributing to this deficit is the dysregulation of translational responses induced by excessive mGluR activation (Muddashetty et al., 2007), disruption of PSD-95 and CAMKII activities (Zalfa et al., 2007) and/or by the abnormal morphology of dendritic spines seen in FXS (Comery et al., 1997; Bilousova et al., 2009). On the other hand, since the loss of FMRP has an impact in the activity of a vast number of proteins, we cannot rule out the possibility that NMDAR-independent mechanisms are also contributing to the alterations in dendritic development found in our study. Regardless, the fact that we did not observe significant reductions in dendritic complexity and NMDAR function in the younger population of granule cells, suggests that the effects of FMRP on NMDAR function and dendritic complexity may occur over time. The DG offers a unique opportunity to study developmental changes, as it is one of the brain areas that exhibits continual neurogenesis throughout the lifespan. Indeed, the loss of FMRP also alters hippocampal neurogenesis in adult animals, which show increased cell proliferation, but impaired neuronal differentiation (Luo et al., 2010; Guo et al., 2012). Together these findings may suggest that the system is trying to compensate for reduced synaptic signaling by enhancing the production of new cells, but that loss of FMRP also negatively impacts the development of these cells. Our data indicate that a loss of FMRP causes significant deficits in both NMDAR function and dendritic arborization in mature neurons, and this could contribute to the abberant neurogenic process and reduced cognitive performance that has been observed with the loss of FMRP.