FMRP, FXR1 protein and Dlg4 mRNA, which are associated with fragile X syndrome, are involved in the ubiquitin–proteasome system
By Hideo Shimizu, and Hirohiko Hohjoh
Excerpt from the article published in Scientific Reports 13, 1956, 02 February 2023, DOI: https://doi.org/10.1038/s41598-023-29152-4
Editor’s Highlights
- Fragile X syndrome (FXS) is an inherited mental and intellectual development disorder and frequently shows autistic spectrum as well.
- The responsible gene for FXS is the fragile X mental retardation 1 (FMR1) gene on chromosome Xq27.3, and an aberrantly expanded CGG repeat (> 200 repeats) in the 5’ untranslated region (UTR) of FMR1 causes the deficiency of the FMR1 protein (also called as FMRP), resulting in the onset of FXS.
- FMR1 and the FMR1 autosomal homolog 1 (FXR1) and 2 (FXR2) genes belong to the fragile X-related (FXR) gene family.
- Discs large MAGUK scaffold protein 4 (DLG4: also called as postsynaptic density protein 95, PSD95) is a major scaffold protein in postsynaptic density and plays an important role in synaptic function.
- FMRP is a carrier of Dlg4 mRNA, binding to and stabilizing the mRNA and regulating its translation.
- FMRP, FXR1 and Dlg4 mRNA as a UPS-related factor.
- The ubiquitin–proteasome system (UPS), by which unwanted and poorly synthesized proteins are systematically degraded, is essential for maintaining homeostasis in vivo, and its dysfunction is thought to cause various impairments such as disease development.
Abstract
The ubiquitin–proteasome system (UPS) is a proteolytic pathway that is essential for life maintenance and vital functions, and its disruption causes serious impairments, e.g., disease development. Thus, the UPS is properly regulated. Here we show novel UPS-related factors: the fragile X mental retardation 1 (FMR1) and Fmr1 autosomal homolog 1 (FXR1) proteins and discs large MAGUK scaffold protein 4 (Dlg4) mRNA, which are associated with Fragile X syndrome, are involved in UPS activity. Fmr1-, Fxr1– and Dlg4-knockdown and Fmr1– and Fxr1-knockdown resulted in increased ubiquitination and proteasome activity, respectively. FXR1 protein was further confirmed to be associated with proteasomes, and Dlg4 mRNA itself was found to be involved in the UPS. Knockdown of these genes also affected neurite outgrowth. These findings provide new insights into the regulatory mechanism of the UPS and into the interpretation of the pathogenesis of diseases in which these genes are involved as UPS-related factors.
Introduction
For quality control of proteins in cells, unwanted proteins and poorly synthesized proteins are actively degraded. The ubiquitin–proteasome system (UPS) is the primary ubiquitin-dependent proteolytic pathway. Unwanted proteins are labelled with ubiquitin by the ubiquitination system, in which ATP-dependent E1 ubiquitin-activating enzyme, E2 ubiquitin-conjugating enzyme, and E3 ubiquitin ligase are involved1,2. Ubiquitinated proteins are rapidly and selectively degraded by proteasomes. The UPS plays an essential role in various biological functions such as cell cycle progression, cell differentiation, signal transduction as well as intracellular protein homeostasis1,2. Thus, abnormalities in the UPS most likely cause serious impairments, for example, disease development such as neurodegenerative diseases1,3,4,5,6.
Fragile X syndrome (FXS) is an inherited mental and intellectual development disorder and frequently shows autistic spectrum as well7. The responsible gene for FXS is the fragile X mental retardation 1 (FMR1) gene on chromosome Xq27.3, and an aberrantly expanded CGG repeat (> 200 repeats) in the 5’ untranslated region (UTR) of FMR1 causes the deficiency of the FMR1 protein (also called as FMRP), resulting in the onset of FXS7. The mutant FMR1 gene with 50–200 CGG repeats also causes Fragile X-associated tremor/ataxia syndrome (FXTAS), a late-onset neurodegenerative disorder that includes cerebellar ataxia, Parkinsonism, memory and executive dysfunction, autonomic dysfunction and other cognitive declines7,8.
FMR1 and the FMR1 autosomal homolog 1 (FXR1) and 2 (FXR2) genes belong to the fragile X-related (FXR) gene family9. These genes encode RNA-binding proteins that have high sequence similarity in functional domains, e.g., the K homology (KH) and RGG box domains for RNA binding9. FMRP is highly expressed in the brain and is thought to play an important role in the translational regulation of several messenger RNAs (mRNAs) that bind to FMRP in postsynaptic neurons, suggesting its contribution to synaptic function such as synaptic plasticity9.
The FXR1 protein is ubiquitously expressed and is known to have an important role in the development of cardiac and skeletal muscle9,10. FXR1 forms heterodimers with FMRP11, and these proteins presumably share a common mRNA target12, shuttle between the nucleus and cytoplasm13 and associate with polyribosomes14. FXR1, like FMRP, may be associated with FXS9. In addition, the associations of FXR1 with other neurological disorders such as bipolar disorder and schizophrenia, and with heart and muscle diseases and carcinogenesis have been reported9,10. However, the functional mechanism of FXR1 remains poorly understood.
Discs large MAGUK scaffold protein 4 (DLG4: also called as postsynaptic density protein 95, PSD95) is a major scaffold protein in postsynaptic density and plays an important role in synaptic function15. Dlg4 mRNA is known to be transported from the cell body to post-synapses and translated there16. FMRP is a carrier of Dlg4 mRNA, binding to and stabilizing the mRNA and regulating its translation16.
Little is known about the relationship between the UPS and the FXR and/or Dlg4 genes, but we have found close relationship among them in this study. Knockdown of Fmr1, Fxr1 and Dlg4 increased ubiquitination levels, and knockdown of Fmr1 and Fxr1 increased proteasome activity. In addition, an association between FXR1 and proteasome was also found. These results suggest that the genes are involved in the UPS. Furthermore, knockdown of these genes was showed to inhibit neurite outgrowth in neuronal cells. This suggests that the UPS may contribute to neurite outgrowth. The findings provide new insights into the regulation of the UPS and the pathogenesis of diseases in which Fmr1, Fxr1 and Dlg4 are involved as UPS regulators.
Results
Reduced GFP expression under Dlg4– and Fxr1-knockdown conditions
We observed that the green fluorescence protein (GFP), examined as a reporter, was reduced in Neuro2a (N2a) cell, a mouse neuroblastoma cell line, where Dlg4 and Fxr1 were knocked down (Fig. 1a and Supplementary Fig. 1). This provided a clue to discovering a novel relationship between the ubiquitin–proteasome system (UPS) and the FXR and Dlg4 genes. In addition, the following points should be noted: N2a cells express Dlg4 mRNA, but the DLG4 protein is hardly detectable, suggesting that Dlg4 mRNA is translationally suppressed in the cells (Supplementary Fig. 2).
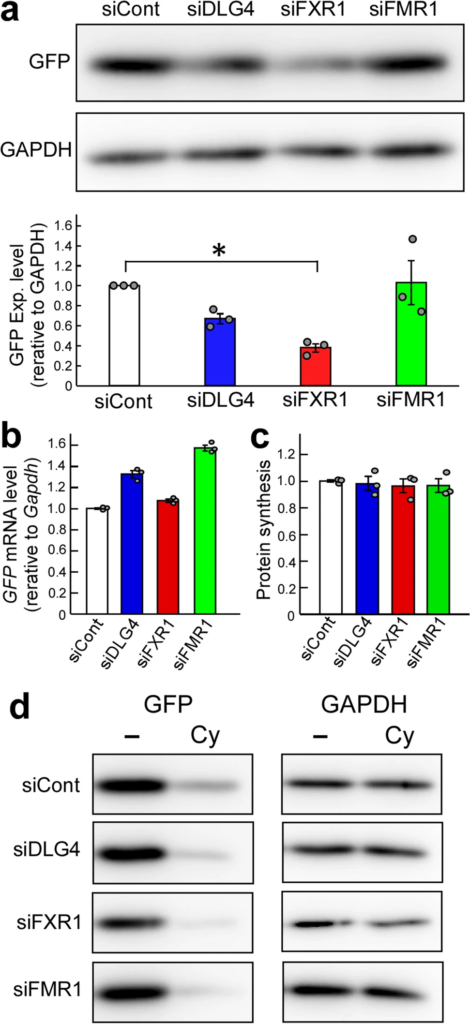
GFP expression under Dlg4-, Fxr1– and Fmr1-knockdown conditions. (a) GFP expression. The pd2EGFP-N1 plasmid encoding the GFP gene was transfected into N2a cells with the indicated siRNAs. siCont: a non-silencing control siRNA. 48 h after transfection, cell lysate was prepared and divided into two portions for western blotting and qRT-PCR analyses. Protein concentrations were measured, and equal amounts of lysates were examined by western blotting with anti-GFP and GAPDH antibodies. The experiment was triplicated (the other two blots are shown in Supplementary Fig. 1), and signal intensities of GFP and GAPDH bands were measured with the ImageJ software. GFP intensities were normalized by GAPDH intensities and further normalized by the data obtained from cells treated with siCont as 1. Data are shown as mean ± SEM (n = 3 examinations, *P < 0.05 by one-way analysis of variance with Dunnett’s post hoc tests). (b) GFP transcript amount. Total RNAs were extracted from the same N2a cells as in (a), and the GFP and Gapdh transcripts were examined by qRT-PCR. The data were analyzed by the delta-delta Ct method using the Gapdh data as a reference and normalized by the data obtained from cells treated with siCont as 1. Data are shown as mean ± SEM (n = 3 independent determination). (c) Protein synthesis level. Overall nascent proteins under gene knockdown conditions (indicated) were examined by a protein synthesis assay kit. Fluorescently labeled nascent proteins were measured and signal intensities were averaged. The data were further normalized by the data obtained from cells treated with siCont. Data are shown as mean ± SEM (n = 3 independent determination). (d) Protein stability. Protein stability was examined using cycloheximide as a translation inhibitor. N2a cells transfected as in (a)were treated with cycloheximide (Cy) and its vehicle (-) (DMSO) for 9 h and examined by western blotting as in (a).
The GFP gene is driven by the CMV promoter in the pd2EGFP-N1 plasmid used. Assuming that gene expression would change depending on the state of N2a cells undergoing gene silencing (knockdown), the GFP transcription and protein synthesis (translation) levels were examined by quantitative RT-PCR (qRT-PCR) and using a protein synthesis assay kit that labels and detects overall nascent proteins in the cells, respectively. As a result, sufficient amounts of GFP transcripts (Fig. 1b) and similar amounts of labeled nascent proteins (Fig. 1c) were detected among the cells examined, suggesting little difference in transcription and translation levels among the cells.
Post-translational regulation of GFP
The protein stability of GFP was examined in Dlg4- and Fxr1-knockdown cells. GFP-expressing N2a cells with Dlg4- and Fxr1-knockdown were treated with cycloheximide, a protein synthesis inhibitor, and GFP and endogenous GAPDH were examined by western blotting. 9-h cycloheximide treatment reduced the GFP level. But, interestingly, the GFP signal intensity of Dlg4- and Fxr1-knockdown cells, as well as Fmr1-knockdown cells, after cycloheximide treatment was even more reduced compared to that of siCont (non-silencing siRNA) and cycloheximide-treated cells (Fig. 1d). The lowest intensity of GFP was observed in Fxr1-knockdown cells after cycloheximide treatment. The signal intensity of endogenous GAPDH also appeared to be reduced in Fxr1-knockdown cells with cycloheximide treatment compared to that in siCont-treated cells. These results, together with the above results, suggest that Dlg4- and Fxr1-knockdown N2a cells possess a lower level of protein stability than intact N2a cells. Thus, Dlg4 and Fxr1 may be related to post-translational regulation.
Increased ubiquitination and proteasome activity by Dlg4-, Fxr1– and Fmr1-knockdown
The UPS is a major pathway that regulates intracellular proteolysis1,2. Dlg4- and Fxr1-knockdown N2a cells, which showed a marked decrease in GFP (Fig. 1a), were treated with MG132, a proteasome inhibitor, and the GFP level was examined. MG132 treatment increased GFP levels, suggesting that proteasome is involved in GFP stability (Supplementary Fig. 3).
We examined whether Dlg4-, Fxr1- and Fmr1-knockdown affects intracellular ubiquitination using an ELISA, which detects and quantifies total intracellular polyubiquitinated proteins. In Dlg4-, Fxr1- and Fmr1-knockdown N2a cells, ubiquitinated protein levels were increased (Fig. 2a). However, 48 h-Fxr1-knockdown cells, in contrast to 24 h-Fxr1-knockdown cells, showed ubiquitination levels comparable to control cells, which will be discussed later.

Ubiquitination and proteasome activity. (a) Ubiquitinated protein levels. N2a cells were transfected with the indicated siRNAs and incubated for 24 or 48 h. The level of total polyubiquitinated proteins in the cells was measured by ELISA. Briefly, cell extracts were prepared, and protein concentrations were determined. Equal amounts of samples (approximately 1.8 µg/µl) were subjected to testing by ELISA, and further examined by western blotting with anti-GAPDH antibody. The ELISA data were normalized with the data obtained from cells treated with siCont as 1. Data are shown as mean ± SEM (n = 3 independent determination, *P < 0.05 by one-way analysis of variance with Dunnett’s post hoc tests). (b) Proteasome activity. N2a cells were treated as in (a). After 48-h gene knockdown, cell lysates were prepared, and protein concentrations were measured. Proteasomes were isolated from equal amounts of lysate (approximately 300 µg) by precipitation with UbL- and control-resin, and their activity was examined by an assay kit. The fluorescent signal produced from a fluorescent-labeled substrate by proteasome activity was measured. Data with UbL-resin were subtracted by data with control-resin (background value) and plotted at arbitrary units (a.u.). Data are shown as mean ± SEM (n = 3 independent determination, *P < 0.05 by two-way analysis of variance). (c)Isolated proteasomes. Isolation of proteasomes was performed as in (b). Concentration-adjusted N2a cell lysates for proteasome preparation (input) and isolated proteasomes (UbL-resin) were examined by western blotting for PSMA7, an essential subunit of the 20S proteasome complex. (d) P62 levels. N2a cells were subjected to gene knockdown as in (a), incubated for 44 h and treated with (+) and without (-) rapamycin (Rm), an inducer of autophagy, for 4 h. Intracellular p62 levels were examined by ELISA. Cell extracts were prepared, and protein concentrations were measured. Equal amounts of samples (approximately 700 ng/µl) were examined by ELISA. Data are shown as mean ± SEM (n = 3 independent determination, *P < 0.05 by one-way analysis of variance with Dunnett’s post hoc tests).
Next, we examined the activity of proteasomes isolated from Dlg4-, Fxr1- and Fmr1-knockdown N2a cells. The results were interesting: proteasome activity was significantly increased in Fxr1- and Fmr1-knockdown cells compared to siCont-treated cells (Fig. 2b). In contrast, the proteasome activity in Dlg4-knockdown cells was the same, i.e., unchanged, as in siCont-treated cells.
Ubiquitinated proteins are degraded not only by proteasome, but also by autophagy involving the p62/SQSTM1 (p62) protein, an adaptor that can bind ubiquitinated proteins17. In particular, aggregates of ubiquitinated proteins are trapped by p62 and are selectively degraded by autophagy. Thus, the level of p62 was examined in Dlg4-, Fxr1- and Fmr1-knockdown cells, and found to be significantly increased in the cells (Fig. 2d); the highest level was observed in Fxr1-knockdown cells. The increased p62 was markedly reduced by treatment with rapamycin (Fig. 2d), an inhibitor of mTOR (mammalian target of rapamycin), which can induce autophagy, suggesting that autophagy was functioning normally in the cell. Therefore, increased ubiquitinated protein aggregates, which are trapped by p62 and subjected to autophagic proteolysis, are thought to accumulate through a rate-limiting proteolytic reaction in autophagy. In any case, the finding is consistent with and supportive of increased ubiquitination by Dlg4-, Fxr1- and Fmr1-knockdown.
As for the observed low level of ubiquitination in 48 h-Fxr1-knockdown cells (Fig. 2a), it is possible that p62-bound ubiquitinated protein aggregates which accumulated intracellularly may be difficult to detect with the assay used, while non-aggregated ubiquitinated proteins, which are detectable by the assay, were rapidly degraded by the enhanced proteasome and significantly reduced in the cells. To confirm this possibility, further studies are needed.
In addition to the above findings, as a contrasting result, data on ubiquitination and proteasome activity in Prnp (Prion protein) and Atf4 (Activating transcription factor 4) knockdown cells were shown to be almost comparable to the data of control cells (Supplementary Fig. 4). These suggest that the increase in ubiquitination and proteasome activity is not triggered by arbitrary gene silencing (knockdown). In other words, there is specificity in gene knockdown that affects the UPS.
When taken together, the findings strongly suggest that Dlg4-, Fxr1- and Fmr1-knockdown affects UPS activity, i.e., Dlg4, Fxr1 and Fmr1 are involved in the UPS.
Effects of Dlg4– and Fxr1-knockdown on UPS activity in the absence of FMRP
The FXR1 protein and Dlg4 mRNA are known to be associated with FMRP11,16. It is of interest whether the effect of Dlg4- and Fxr1-knockdown on UPS activity is dependent on FMRP. To see the effect of Dlg4- and Fxr1-knockdown on the UPS in the absence of FMRP, we generated a FMRP-deficient N2a cell line (named def.FMRP-N2a cell) by gene editing with the CRISPR-Cas9 system (Supplementary Fig. 5a); and def.FMRP-N2a cells were subjected to gene silencing for Dlg4 and Fxr1 followed by ubiquitination and proteasome activity assays as above. The results indicated that ubiquitination and proteasome activity were increased in the cells with Dlg4- and Fxr1-knockdown and in Fxr1-knockdown cells, respectively (Supplementary Fig. 5b,c), which are consistent with the results using naïve N2a cells (see Fig. 2). Thus, Dlg4- and Fxr1-knockdown promotes ubiquitination independently of FMRP, and FXR1 is related to proteasome activity independently of FMRP. In addition to the above double-gene suppression conditions, a combination of Fxr1- and Dlg4-knockdown was also examined. As a result, in both ubiquitination and proteasome assays, the results of double-gene knockdown cells were similar to those of Fxr1 single-knockdown cells, i.e., there were few additive and synergistic effects in the double-gene knockdown cells (Supplementary Fig. 6). This suggests that Fxr1-knockdown may have a greater impact on the UPS than Dlg4-knockdown.
Association between FXR1 protein and Dlg4 mRNA
Based on the amino acid sequence homology between FXR1 and FMRP, FXR1 is expected to bind to Dlg4 mRNA as well as FMRP. The binding of FXR1 to Dlg4 mRNA was examined in the absence of FMRP effects, i.e., using def.FMRP-N2a cells. RNAs coimmunoprecipitated with anti-FXR1 antibody from def.FMRP-N2a cell extracts were examined by qRT-PCR for Dlg4. The results indicated the presence of Dlg4 mRNA in the precipitants, suggesting that Dlg4mRNA is associated with FXR1 protein (Fig. 3).
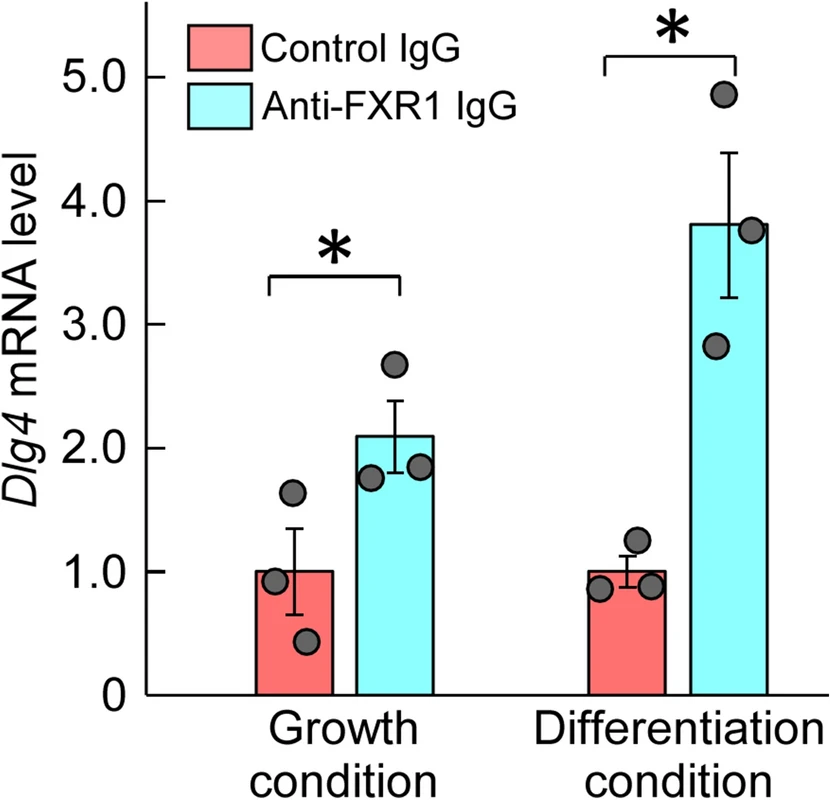
Association between FXR1 protein and Dlg4 mRNA. Immunoprecipitation with anti-FXR1 antibody was performed in def.FMRP-N2a cells (in the absence of FMRP) cultured under normal growth and retinoic acid (RA)-added differentiation conditions. RNA was extracted from the precipitants with anti-FXR1 IgG or control IgG and examined by qRT-PCR for Dlg4. The data were normalized by the data of control IgG as 1. Data are shown as mean ± SEM (n = 3 independent determination, *P < 0.05 by one-tailed t-test).
FXR1 binds to proteasome, but FMRP does not
The findings so far raised an important question: do FMRP and FXR1 bind to proteasome? To address the question, we collected proteasomes from cells using the UbL-Resin containing the ubiquitin-like domain and examined them by western blotting for FMRP and FXR1. The results were intriguing: FXR1 was found to be present in the collected proteasomes, but FMRP was not detected (Fig. 4a). This strongly suggests that FXR1 protein binds to proteasomes, but FMRP does not.

Association of FXR1 protein with proteasome. (a) Association between FXR1 and proteasome. Proteasomes were collected by precipitation with the UbL-resin and Control-resin from N2a cells cultured under growth conditions (FBS +) and serum-free differentiation conditions with (FBS-/RA +) or without (FBS-/RA-) retinoic acid. The collected proteasomes were examined by western blotting for FXR1, FMRP and PSMA7 (an essential subunit of the 20S proteasome complex). (b) Association of FXR1 with proteasome under Dlg4-knockdown conditions. Proteasomes were collected from N2a cells treated with siDLG4 and siCont, and examined by western blotting for FXR1 as in (a).
We further examined whether FXR1 can bind to proteasomes even under Dlg4- knockdown conditions; this is because Dlg4 mRNA is associated with FXR1 (Fig. 3). The results showed that FXR1 coprecipitated with proteasomes even under Dlg4- knockdown conditions (Fig. 4b), suggesting that Dlg4 mRNA may not mediate FXR1 binding to proteasomes.
Effects of Dlg4-, Fxr1– and Fmr1-knockdown on process formation and UPS activity under differentiation conditions
N2a cell can form neurite-like processes under serum-free and retinoic acid (RA)-added culture conditions (differentiation conditions)18. When Dlg4, Fxr1 and Fmr1 were knocked down, the resulting N2a cells suppressed process formation under serum-free differentiation conditions (Fig. 5a). However, supplementation with RA restored process formation in Fmr1-knockdown N2a cells, but not in Dlg4- and Fxr1-knockdown cells (Fig. 5a). Similarly, in def.FMRP-N2a cells, process formation occurs under RA-added differentiation conditions but is cancelled by Dlg4- and Fxr1-knockdown (Fig. 5b).
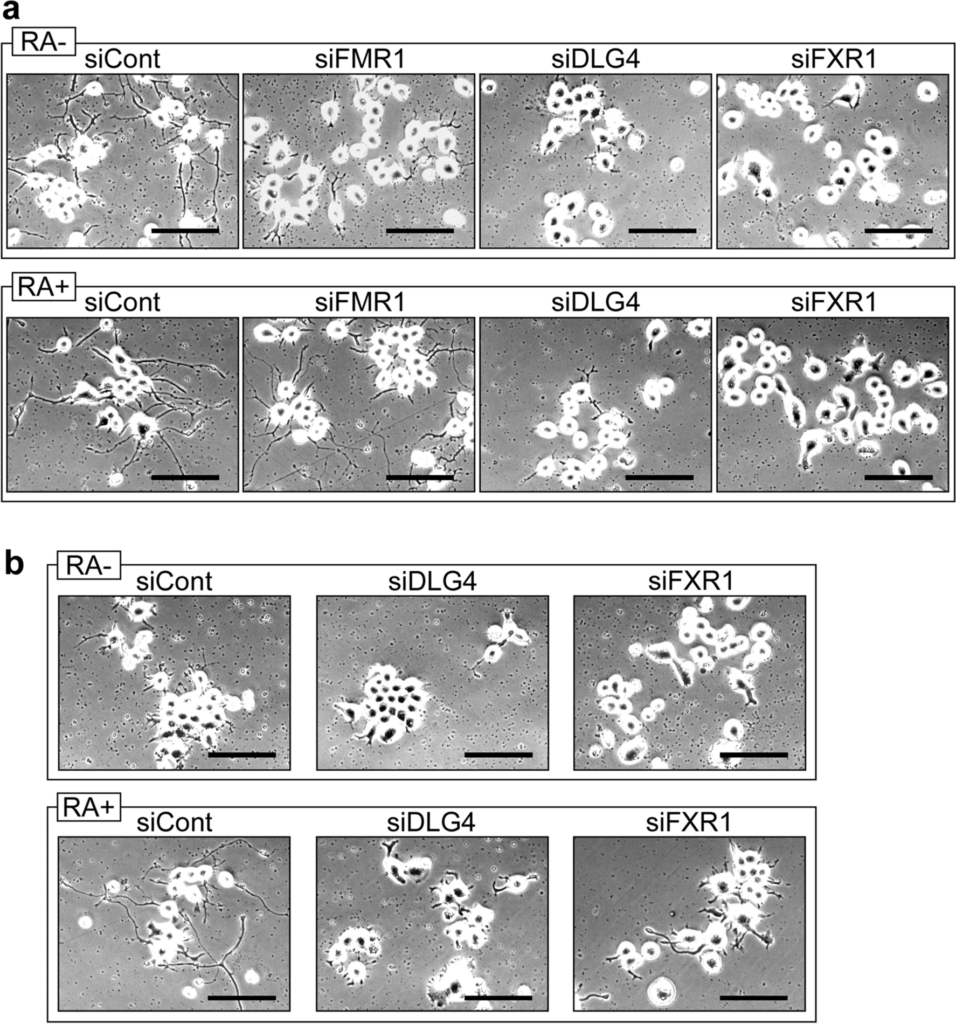
Process formation of N2a cell. (a) N2a cells with gene knockdown under differentiation conditions. N2a cells were transfected with the indicated siRNAs and incubated in serum-free differentiation medium with (RA +) or without (RA-) retinoic acid for 3 days. The cells were observed by a microscope. Scale bars indicate 100 μm. (b) Def.FMRP-N2a cells with gene knockdown under differentiation conditions. Def.FMRP-N2a cells were treated and observed as in (a). Scale bars indicate 100 μm.
UPS activity was examined in N2a cells under differentiation conditions. As shown in Supplementary Fig. 7a, ubiquitination was increased, but proteasome activity remained unchanged except for a slight decrease under serum-free differentiation conditions. Similar results were also obtained in def.FMRP-N2a cells under differentiation conditions (Supplementary Fig. 7b).
The effects of Dlg4-, Fxr1– and Fmr1-knockdown on UPS activity under differentiation conditions were examined. Gene knockdown of Dlg4 and Fmr1 (for 48 h) increased ubiquitination under differentiation conditions, and Fxr1– and Fmr1-knockdown significantly increased proteasome activity (Supplementary Fig. 7c,d). These results are consistent with the results of N2a cells under growth culture conditions (Fig. 2). Consequently, the effects of Dlg4-, Fxr1– and Fmr1-knockdown on UPS activity do not appear to change under either growth culture or differentiation conditions.
Effects of Dlg4– and Fxr1-knockdown on neurite outgrowth
The inhibition of process formation in N2a cells with Dlg4- and Fxr1-knockdown is particularly striking (Fig. 5). To determine whether the gene knockdown also affects actual neurite outgrowth, primary mouse neurons were prepared and transfected with Dlg4 and Fxr1 siRNAs together with the GFP gene as a reporter; and neurite outgrowth was examined in GFP-positive neurons. As expected, Dlg4- and Fxr1-knockdown markedly suppressed neurite outgrowth of GFP-positive neurons (Fig. 6a). We further examined the expression of the target genes (Dlg4 and Fxr1) in the cultured neurons. The results of Dlg4 are noteworthy: early in the culture, when neurite outgrowth is active (see siCont-treated neurons in Fig. 6a), Dlg4 mRNA was present in sufficient amounts in neurons, but little DLG4 protein was detected (see day 1 and day 4 in Fig. 6b). In contrast, Fxr1 mRNA and the protein were detected in sufficient amounts throughout the culture period. These results are consistent with those obtained in N2a cells (Supplementary Fig. 2a). Thus, Dlg4 mRNA is expressed at sufficient levels during neurite outgrowth but is translationally suppressed. This together with the impact of Dlg4-knockdown suggest that Dlg4 mRNA itself has a function in neurons during neurite outgrowth.
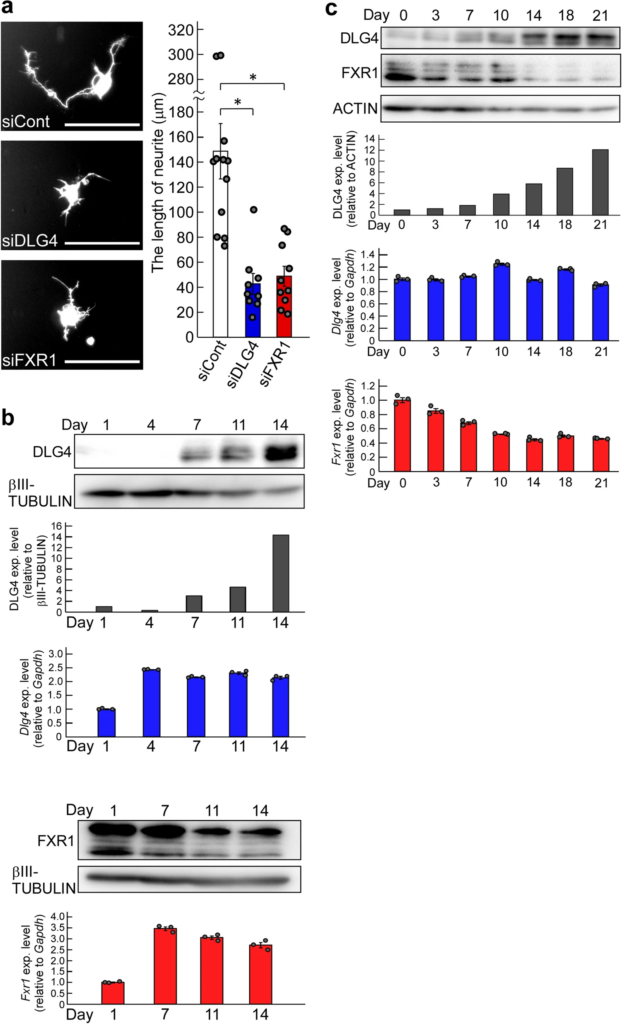
Effects of Dlg4– and Fxr1-knockdown on neuritogenesis, and the gene expression profiles. (a) Effects of Dlg4– and Fxr1-knockdown on neurite outgrowth. The day (day 0) before transfection, primary mouse neurons were prepared and seeded onto culture plates. Transfection of the indicated siRNAs together with the GFP gene as a reporter was performed (day 1). 3 days after transfection (day 4), GFP-positive neurons were examined, and representative neurons were exhibited (see photos). Scale bars indicate 100 μm. The longest neurite of each neuron was measured and at least 9 neurons were examined in each siRNA-transfected neuron (see graph). The data were compared to the data obtained from siCont-transfected neurons (*P < 0.05 by one-way analysis of variance with Dunnett’s post hoc tests). Data are shown as mean ± SEM. (b) Expression of Dlg4 and Fxr1 during culture of primary neurons. Day 1 is the day after the preparation and seeding of primary neurons as in (a). Proteins and total RNAs were extracted from neurons on the indicated day and examined by western blotting and qRT-PCR, respectively. In western blot, DLG4, FXR1 and βIII-TUBULIN (as a loading control) were examined. Signal intensities of DLG4 and βIII-TUBULIN bands were measured with the ImageJ software. DLG4 intensities were normalized by βIII-TUBULIN intensities and further normalized by Day1 data as 1 (black bar-graph). The qRT-PCR data of Dlg4 (blue bar-graph) and Fxr1 (red bar-graph) were analyzed by the delta-delta Ct method using the data of Gapdh as a reference and further normalized by the data of Day 1 as 1. Data are shown as mean ± SEM (n = 3 independent determination). (c) Expression profiles of Dlg4 and Fxr1 during postnatal mouse brain development. Proteins and total RNAs were extracted from the brain at the indicated postnatal day (Day 0 is delivery day) and examined as in (b). In western blot, ACTIN was examined as a loading control. DLG4 intensities were normalized by ACTIN intensities and further normalized by Day0 data as 1 (black bar-graph).
Neurite elongation and synapse formation are essential for postnatal brain development. We investigated the expression profiles of Dlg4 and Fxr1 over the course of postnatal mouse brain development. As shown in Fig. 6c, sufficient amounts of Dlg4 mRNA were detected during postnatal brain development, but only small amounts of DLG4 protein were present during the first few days, after which DLG4 protein gradually increased. In contrast, Fxr1mRNA and the protein, which correlate with each other, were expressed in sufficient amounts at birth, gradually decreased, and stabilized after postnatal day 14 (Fig. 6c). These results are consistent with the in vitro results (Fig. 6b and Supplementary Fig. 2) and support an important role for Dlg4 mRNA in neurite outgrowth during postnatal brain development.
Discussion
The UPS, by which unwanted and poorly synthesized proteins are systematically degraded, is essential for maintaining homeostasis in vivo, and its dysfunction is thought to cause various impairments such as disease development1,2. The UPS is properly regulated, and its regulation is complex. For example, various E3 ubiquitin ligases and their substrate specificities represent some of the complexity2. Elucidating the regulatory mechanisms of the UPS is vital for understanding homeostatic mechanisms based on protein quality control. In this study, we have shown that the FXR and Dlg4 genes, which are related to Fragile X syndrome (FXS)9,19, are associated with the UPS. Knockdown of these genes promote UPS activity (Fig. 2). Furthermore, we have found that FXR1 protein is associated with proteasome (Fig. 4), and that Dlg4 mRNA, but not the protein, is implicated in the UPS (Fig. 2 and Supplementary Fig. 2). Although the regulatory mechanism of the UPS, such as the substrate specificity of the E3 ubiquitin ligase involved in selective proteolysis2, are known about the specificity of UPS, the factors presumed to regulate UPS activity as shown here may be unique.
FMRP is an RNA binding protein that is known to be involved in mRNA transport and localization in neuronal cells and in protein synthesis at the synapse7,9. FMRP plays a variety of roles in neuronal cells, but its function has not been fully understood. The deficiency of FMRP causes FXS, to which aberrantly expanded CGG repeat in the 5’ UTR of Fmr1 is closely related7,9. The loss of FMRP does not result in neuronal cell death, but rather in neurodevelopmental abnormalities20,21. FMRP-knockdown and -knockout N2a cells are similarly non-lethal, but process formation depends on the presence or absence of retinoic acid in culture conditions. This may reflect the vulnerability of FMRP-deficient cells to neuritogenesis. Deficient FMRP is thought to cause dysregulation of the translation of mRNAs that bind to FMRP, which in turn causes various manifestations of FXS.
This study adds new insights into the interpretations of the pathogenesis of FXS; that is, dysregulation of the UPS due to FMRP deficiency may be involved in the pathogenesis of FXS. Accumulation of ubiquitinated protein aggregates has been observed in the brain of FXTAS22,23 and in neurons of Fmr1-knockout mice21. The observed increase in ubiquitination due to FMRP deficiency in this study (Fig. 2) is consistent with the previous observations. Thus, these suggest that FMRP deficiency affects the UPS.
The remaining issue in understanding the dysregulation of UPS due to FMRP deficiency is that the degradation capability in relation to the accumulation of ubiquitinated proteins needs to be studied extensively, including not only proteasome activity but also autophagy and levels of ubiquitination.
FXR1 has been reported to be associated with various diseases as well as vital functions9,10, but its functional mechanism has not yet been fully elucidated. Since FXR1 is an RNA binding protein similar to FMRP9, RNA metabolism and translational regulation by RNA binding of FXR1 may be involved in the functional mechanism. In this study, we have found that FXR1 binds to proteasome and that proteasome activity is increased in the absence of FXR1 (Figs. 2, 4), suggesting that FXR1 plays an important role in proteasome regulation. In addition, it also appeared to be involved in intracellular ubiquitination (Fig. 2). Since little was known about the relationship between FXR1 and the UPS, these findings are unexpected and provide us with new insight into the functional mechanism of FXR1. Furthermore, reconsidering FXR1 as a UPS-related factor may provide a new interpretation of the pathogenesis of FXR1-associated diseases. For example, protein quality control problems caused by FXR1 dysfunction may be involved in FXR1-associated diseases. Further progress and studies are expected to elucidate new functional mechanisms of FXR1 as a UPS-related factor in biological functions and pathogenesis in the future.
Another intriguing finding in this study is that the Dlg4 mRNA is functional and involved in the UPS (Fig. 2 and Supplementary Fig. 2). DLG4 (also called PSD95) is a well-known protein involved in synapse formation and functions15. In contrast, little is known about intracellular contribution and function of the Dlg4 mRNA. Thus, the finding was unexpected and attracted our interest. Dlg4 mRNA is thought to bind to a variety of proteins including FMRP and FXR1, and one such Dlg4 mRNA-protein complex may work as a UPS regulator. Dlg4 mRNA may act as a cofactor in such a UPS-related factor, and its (RNA) degradation may cause dysfunction of the factor, thereby leading to poor UPS control. It is of interest to identify protein(s) that bind to Dlg4 mRNA and work as a UPS-related factor. FXR1 may be a candidate because it is associated with Dlg4 mRNA and binds to proteasome (Figs. 3, 4). Further studies exploring candidate proteins, including FXR1, are needed to elucidate Dlg4 mRNA-protein complex that works as a novel UPS-related factor.
Dlg4 mRNA is also involved in neurite outgrowth (Figs. 5, 6). Interestingly, Dlg4 mRNA appears to be expressed but translationally suppressed during early postnatal brain development, when neurite outgrowth is active (Fig. 6b, c). Similarly, in N2a cells, which can form neurite-like processes, Dlg4 mRNA is expressed but the protein is hardly detected (Supplementary Fig. 2). Since gene silencing for Dlg4 significantly suppresses neurite outgrowth and process formation (Figs. 5, 6), Dlg4 mRNA may act as a functional RNA in neuritogenesis. Given that Dlg4 mRNA is translationally suppressed during neurite outgrowth and then translated, it is conceivable that: Dlg4 mRNA itself contributes to neurite outgrowth and the DLG4 protein, released from translational suppression, participates in synapse formation after neurite formation. Thus, it can be said that the Dlg4 gene carries multiple genetic information and functions, as it is both a functional RNA and a template (information carrier) for protein synthesis.
Like Dlg4-knockdown, Fxr1-knockdown also showed a strong inhibition to neurite outgrowth and process formation (Figs. 5, 6), and both Fxr1– and Dlg4-knockdowns affected the UPS (Fig. 2). In neurons, the UPS plays important roles24. Thus, Dlg4 mRNA may be involved in neuritogenesis through the UPS. Further studies are needed to determine whether Dlg4mRNA and FXR1 are involved in neuritogenesis as UPS-related factors.
Unlike Fmr1-, Fxr1- and Dlg4-knockdown, Prnp– and Atf4-knockdown has little impact on the UPS (Supplementary Fig. 4). This indicates that there is specificity in gene knockdown that affects the UPS, i.e., some genes are associated with the UPS and some ones are not. Each such associated gene may contribute to regulation of the UPS by a different mechanism of action. For example, Dlg4-, Fxr1– and Fmr1-knockdown appears to result in different levels of ubiquitination (Fig. 2a). Fxr1– and Fmr1-knockdown increases proteasome activity (Fig. 2b), but FXR1 binds to proteasomes and FMRP does not (Fig. 4). These may provide evidence for different mechanisms of action. Different target proteins may be ubiquitinated by different gene knockdowns, and different E3 ubiquitin ligases may be involved in the ubiquitination; that is, FMRP, FXR1 and Dlg4 mRNA may be associated with different E3 ubiquitin ligases. Furthermore, these UPS-related factors may affect the specificity and reactivity of proteolysis not only in proteasome but also in autophagy. These are of importance for understanding their mechanisms of action and need to be verified in the future.
Finally, this study has revealed a new role for FMRP, FXR1 and Dlg4 mRNA as a UPS-related factor. These molecules are known to be involved in diseases and vital functions, but their association with the UPS was unknown. Therefore, we expect that this discovery will provide new clues to the investigation of the underlying causes of diseases in which these genes are involved and to the elucidation of their biological functions.

