Activation of the sigma-1 receptor ameliorates neuronal ferroptosis via IRE1α after spinal cord injury
By Rui Tan, Chunxiao Sui, Yuhang Diao, Guihong Shi, Xiaojun Hu, Zhenghao Hao, Chenyang Li, Mingyu Hao, Minghao Xie, and Tao Zhu
Excerpt from the article published in Brain Research, May 18 2024, 149011, ISSN 0006-8993, DOI:
https://doi.org/10.1016/j.brainres.2024.149011.
Highlights
- Our study found that Sig-1R activation effectively promoted the recovery of motor function in mice after spinal cord injury, attenuated neuronal apoptosis, reduced the production of pro-inflammatory cytokines and iron accumulation, and inhibited ferroptosis in spinal cord tissues after SCI in mice.
- Ferroptosis and IRE1α were significantly upregulated after spinal cord injury, and sigma-1 receptor agonists were able to ameliorate this result through the elimination of inositol-requiring enzyme-1 alpha (IRE1α)-mediated neuronal ferroptosis.
- Therefore, sigma-1 receptor activation could attenuate ferroptosis after spinal cord injury by reducing IRE1α and improve functional recovery after SCI, providing a new therapeutic strategy for the treatment of SCI.
Abstract
Spinal Cord Injury (SCI) is a debilitating disease associated with a significant economic burden owing to its high level of disability; however, current treatment options have only limited efficacy. Past research has shown that iron-dependent programmed cell death, also known as ferroptosis, plays a critical role in the pathogenesis of SCI. The sigma-1 receptor (Sig-1R) is widely distributed in the central nervous system, and has been implicated in the pathophysiology of several neurological and psychiatric disorders. Several in vivo and ex vivo studies have shown that Sig-1R activation exerts unique neuroprotective effects. However, the underlying mechanisms remain unclear. To date, no study has yet demonstrated the association between Sig-1R activation and ferroptosis in patients with SCI. However, the present study found that Sig-1R activation effectively promoted the recovery of motor function in mice after spinal cord injury, attenuated neuronal apoptosis, reduced the production of pro-inflammatory cytokines and iron accumulation, and inhibited ferroptosis in spinal cord tissues following SCI in mice. Ferroptosis and IRE1α were significantly upregulated after spinal cord injury, while sigma-1 receptor agonists were able to facilitate this result through the elimination of inositol-requiring enzyme-1 alpha (IRE1α)-mediated neuronal ferroptosis. Therefore, sigma-1 receptor activation could attenuate ferroptosis after SCI by reducing IRE1α and improving functional recovery after SCI, potentially representing a new therapeutic strategy for treating SCI.
1. Introduction
Spinal cord injury (SCI) is a devastating disease that often results in permanent muscle paralysis, sensory loss, and autonomic dysfunction below the level of injury (McDonald and Sadowsky, 2002). Persistent functional impairment is primarily caused by the limited ability of damaged central nervous system (CNS) neurones to regenerate axons and re-establish functional connections. The spinal cord has a unique structure, resembling an information highway, in which any severe injury at a particular point along the path can block the flow of information in both directions (Tran et al., 2018). SCI has further become a severe public health problem, with approximately 250,000–500,000 new cases diagnosed annually, placing an enormous burden on society and families. The pathogenesis of SCI can be divided into both primary and secondary types. Primary injury is caused by external forces acting directly on the spinal cord or spine, causing mechanical damage to the spinal cord or nerve roots, such as tearing, compression, and shearing. Primary injuries usually occur during trauma and result in spinal cord haemorrhage, oedema, ischaemia, and necrosis. The location and extent of the primary injury determine the type and severity of spinal cord injury, such as tetraplegia or paraplegia. Secondary injury is caused by a series of complex biochemical and pathological reactions triggered by the primary injury, resulting in further damage to the spinal cord. Secondary injury usually occurs within minutes to weeks of trauma, and involves various molecules and cells such as free radicals, calcium ions, inflammatory factors, apoptotic factors, and immune cells. Secondary injury can increase the extent of primary injury, exacerbate the loss of neurological function, and interfere with nerve regeneration and repair (Anjum et al., 2020, de Almeida et al., 2023, Hu et al., 2023, Sterner and Sterner, 2022). Current treatments for SCI include early surgical intervention to relieve spinal cord compression and the use of anti-inflammatory, antioxidant, neurotrophic, and other drugs to reduce secondary injury and protect the enduring nerve function. However, there are currently no drugs which can effectively improve neurological function and prognosis in patients after spinal cord injury (Hashimoto et al., 2024).
Ferroptosis is a form of iron-ion-dependent cell death characterised by lipid peroxidation (Jiang et al., 2021). This pathway is closely associated with the pathophysiology of spinal cord injury. Iron-induced cell death, also known as ferroptosis, has been extensively studied in various diseases, including Alzheimer’s disease and acute brain injury (Ou et al., 2022). Compelling evidence links ferroptosis to secondary injury following SCI. Inhibition of ferroptosis is crucial for reducing neuronal cell death and neuroinflammation in SCI. Compelling evidence links ferroptosis to secondary injury after SCI. Inhibition of ferroptosis is critical for reducing neuronal cell death and neuroinflammation in SCI (Bai et al., 2023, Hu et al., 2021, Li and Jia, 2023). Recent studies have demonstrated that ferroptosis can induce endoplasmic reticulum (ER) stress (Lee et al., 2018).
The Sigma-1 receptor (Sig-1R) is a transmembrane receptor located primarily on the mitochondria-associated endoplasmic reticulum membrane (Meng et al., 2022). In addition to its role as a receptor, Sig-1R also functions as a chaperone. The Sigma-1 receptor (Sig-1R) is involved in cell signalling and the regulation of receptors and ion channels, and has been implicated in the development of various neurodegenerative diseases and cancers (Kim and Maher, 2017). It is widely distributed in the central nervous system, and is involved in the pathophysiological changes associated with neurological and psychiatric disorders (Jia et al., 2018, Shi et al., 2021). Recent research has further shown that the activation of Sig-1R can protect against traumatic neurological injury, making it a potential target for treating SCI. Sig-1R is associated with IRE1 during endoplasmic reticulum stress. IRE1α, a significant component of the IRE1 protein, is the principal executor of ER stress (Gaja-Capdevila et al., 2021). Under conditions of ER stress, IRE1 oligomerizes and activates its ribonuclease activity through autophosphorylation (Sun et al., 2015).
Thus, we hypothesised that modulation of the sigma-1 receptor-IRE1 pathway could enhance iron removal and promote neurological function recovery in mice following SCI.
…
3. Results
3.1. Sigma-1 receptor activation promotes lower limb motor recovery after SCI
SCI injury was induced in mice (Fig. 1B), with FLV injection initiated 2 h after SCI surgery and continued for three days in the FLV group. BMS scores and hindlimb kinematics (Fig. 1C) were used to analyze the recovery of motor function after SCI in mice. Compared with mice in the SCI group, FLV-injected mice recover better hindlimb motor function at 14-, 21-, and 28-days post injection (dpi) (Fig. 1D), which corresponded to higher BMS scores (Fig. 1E). Hindlimb kinematics further showed that, compared with mice in the SCI group, FLV-injected mice with SCI at 28 dpi acquired better motor function (Fig. 1E-F), including a higher leg lift height (Fig. 1F). Compared with the FLV group, mice with SCI injected with FLV + BD-1047 showed a significant deterioration in motor function at 28 dpi (Fig. 1E-F), as well as a decrease in leg lift height (Fig. 1F). Compared with the SCI group, SCI mice injected with BD-1047 showed further deterioration in motor function (Fig. 1D-F). Although the mice in the FLV group showed a better therapeutic effect, their motor function did not recover to the level observed in the uninjured group (Fig. 1D-F). In conclusion, our results suggest that Sig-1R activation by FLV contributes to the recovery of motor function after SCI.
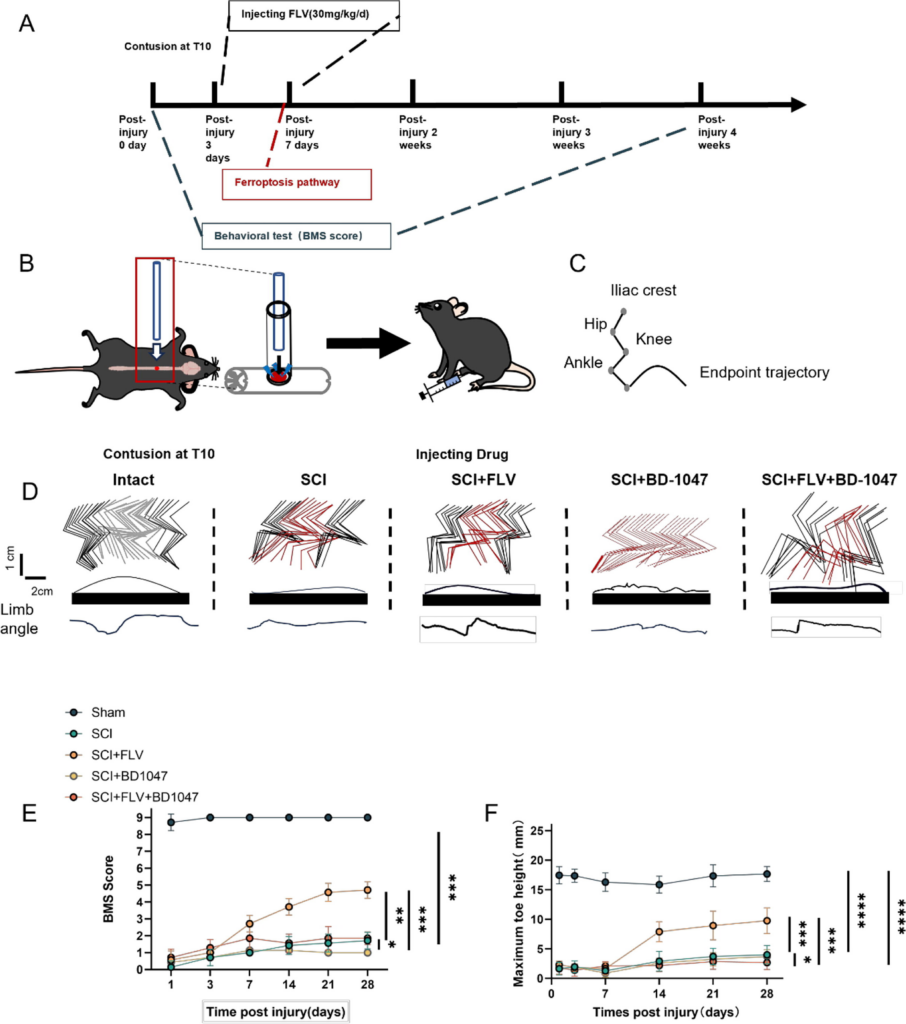
Sigma-1 receptor activation promotes lower limb motor recovery after SCI.
(A) Schedule of drug injection and behavioral assessment. (B) Schematic diagram of spinal cord injury in mice. (C) Schematic diagram of hindlimb kinematics. (D) Hindlimb kinematics at 28 dpi in sham group, SCI group, SCI + FLV group, SCI + BD-1047 group, SCI + FLV + BD-1047 group, representative images of endpoint trajectory and limb angle. (E) Motor function assessed by BMS at 1, 3, 7, 14, 21, and 28 dpi in each group (n = 8). (F) Maximum toe height at 1, 3, 7, 14, 21, and 28 dpi in each group (n = 8). *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 by one-way ANOVA, n = 8 animals per group.
3.2. Activation of the Sigma-1 receptor can inhibit spinal cord ferroptosis and protect neurones
This study aimed to examine the effects of Sig-1R activation on neuronal growth and ferroptosis following SCI. Seven days after SCI, western blot analysis revealed a significant reduction in GPX4 and xCT expression in the SCI group (Fig. 2A). In contrast, FLV-treated SCI mice showed a substantial increase in GPX4 and xCT expression. SCI mice treated with BD-1047 showed further reductions in the expression of GPX4 and xCT. The expression of GPX4 and xCT was attenuated in the FLV + BD-1047 group, which was significantly different from that in the FLV group, but not significantly different from that in the SCI group (Fig. 1A). There was no significant difference in iron death in the spinal cord tissues after SCI between the FLV and SCI groups (Fig. 2A, D, E). The expression of ACSL4 and 4HNE, which represent an increase in ferroptosis, was lower in the FLV group than in the SCI group. However, the expression of these proteins was further increased in SCI mice treated with BD-1047 compared to the SCI alone group. Protein expression was higher in the FLV + BD-1047 group than in the SCI group. Conversely, protein expression was reduced in the FLV + BD-1047 group compared to that in the SCI group. In the FLV + BD-1047 group, protein expression was significantly higher than that in the FLV group and was not substantially different from that in the SCI group (Fig. 2A–C). Neuronal activity and its relationship to ferroptosis were examined by immunofluorescence staining for ACSL4, NeuN, TUNEL, and FJC (Fig. 2F–H). The results showed a significant increase in ACSL4-positive neurones, as well as a decrease in NeuN in the SCI group cord tissue sections of the spinal group (Fig. 2F–H). Compared to the SCI group, the FLV group showed a significant reduction in the number of ACSL4-positive neurones (Fig. 2F), whereas the BD-1047 group showed a significant increase (Fig. 2F, G). The FLV + BD-1047 group had a significantly higher number of ACSL4-positive neurones than the FLV group; however, there was no significant difference in the number of NeuN positive cells between the two groups (Fig. 2F–H). The trend for NeuN was thus the opposite of that for ACSL4.
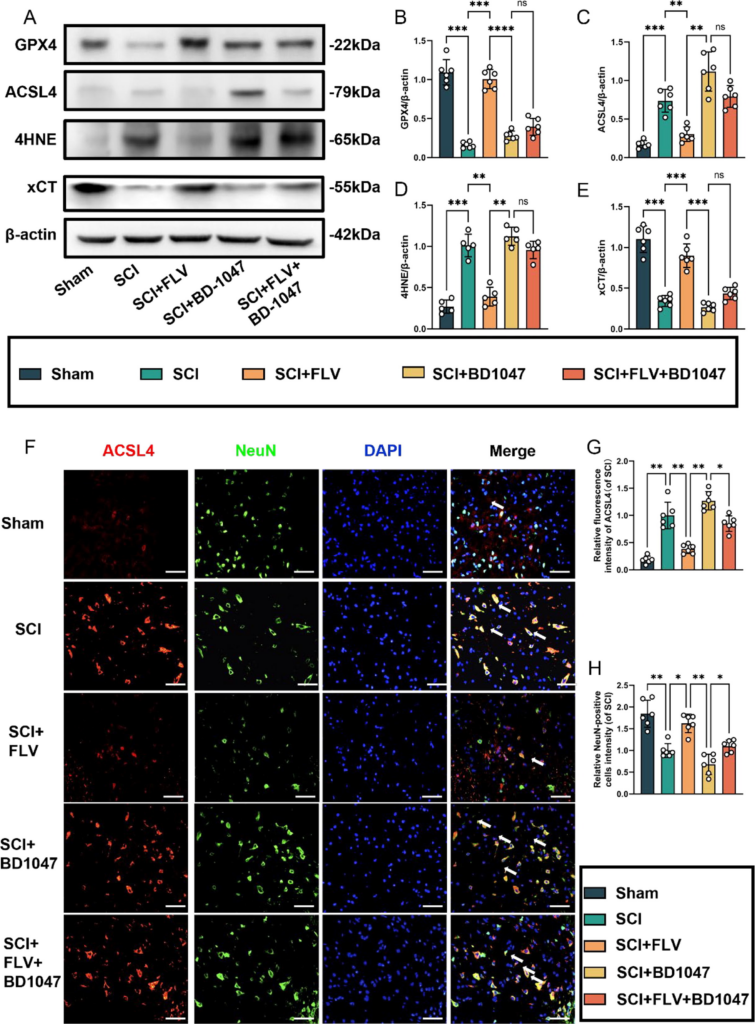
Activation of the Sigma-1 receptor can inhibit spinal cord ferroptosis.
(A) Western blotting was performed to assess the expression of GPX4, ACSL4, 4HNE, and xCT 7 days after SCI. (B-E) Quantitative analysis was conducted on the expression of GPX4, ACSL4, 4HNE, and xCT (n = 6, all data are expressed as mean ± standard deviation using one-way ANOVA). (F) Immunofluorescence staining was used to detect the levels of ACSL4 and NeuN in each group. (G, H) Statistical analysis was conducted on immunofluorescence staining of ACSL4-positive neuronal cells and neuronal cells in each group (of SCI group, n = 6, scale bar = 50 µm, all data are expressed as mean ± SD using one-way ANOVA). The results showed statistical significance with *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 by one-way ANOVA, n = 6 animals per group.
TUNEL staining revealed a significant increase in the number of TUNEL-positive neurones in the SCI group (Fig. 3A–C). The number of TUNEL-positive neurones was further significantly reduced in the FLV group compared to that in the SCI group, whereas it was increased in the BD-1047 group. In the FLV + BD-1047 group, the number of TUNEL-positive neurones was also significantly higher than that in the FLV group, but was not substantially different from that in the SCI group. FJC staining further indicated that the number of FJC-positive neurones in the FLV group significantly decreased, whereas it increased in the BD-1047 group. In the FLV + BD-1047 group, the number of FJC-positive neurones was significantly higher than that in the SCI group. In contrast, the number of FJC-positive neurones decreased in the BD-1047 group, but was not significantly different from that in the FLV + BD-1047 group (Fig. 3D, E). Thus, these results indicate that activation of Sig-1R mitigates ferroptosis in spinal cord tissues and provides neuronal protection.
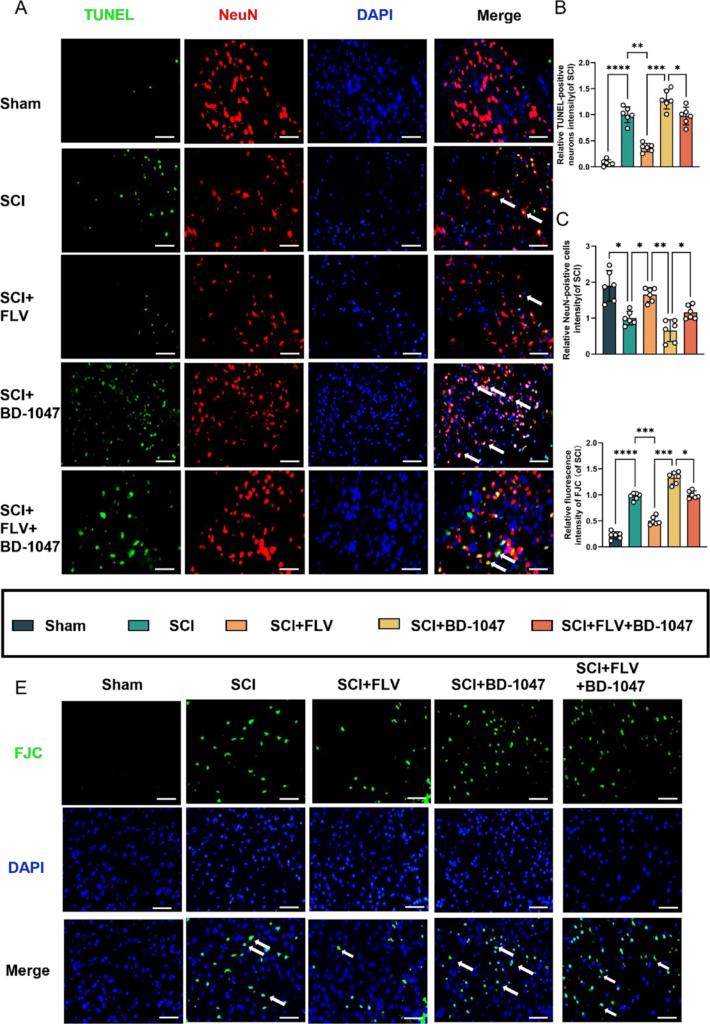
Effects of activation of the Sigma-1 receptor on neuronal death seven days after SCI.
(A) Representative images of TUNEL (green) co-localized with neurones (NeuN, red) in each group after seven days of spinal cord injury (SCI) were detected by immunofluorescence staining. Cell nuclei were stained with DAPI (blue) (scale bar = 50 µm). (B-C) This study analyzed TUNEL-positive neuronal cells and immunofluorescence staining of neuronal cells in each group(of SCI group, n = 6, scale bar = 50 µm, all data are expressed as mean ± SD using one-way ANOVA). (D) The number of FJC-positive cells seven days after SCI was quantitatively analyzed(of SCI group, n = 6, scale bar = 50 µm, all data are expressed as mean ± SD using one-way ANOVA). (E) Representative images of FJC (green) staining seven days after SCI are shown. The statistical analysis showed significant differences with *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 by one-way ANOVA, n = 6 animals per group. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.3. LIP-1 ameliorates the ferroptosis exacerbated by sigma-1 receptor inhibitors
Previous studies have shown that BD-1047, a Sigma-1 receptor inhibitor, exacerbates ferroptosis in spinal cord tissues following SCI. Protein blotting analysis conducted seven days after SCI revealed a significant increase in the expression of GPX4 and xCT in SCI mice treated with LIP-1 (Fig. 4A). The expression of these proteins was higher in the LIP-1 + BD-1047 group than in the BD-1047 group (Fig. 4A, B, E). The expression of ACSL4 and 4HNE was also increased in the SCI group, indicating an increase in ferroptosis after spinal cord tissue injury. In contrast, the expression of these proteins decreased in the LIP-1 group. The expression of these proteins was increased in the spinal cords of mice treated with BD-1047 compared to that in the SCI group. However, in the LIP-1 + BD-1047 group, the expression of these proteins was significantly reduced compared to the BD-1047 group (Fig. 4A, C, D).
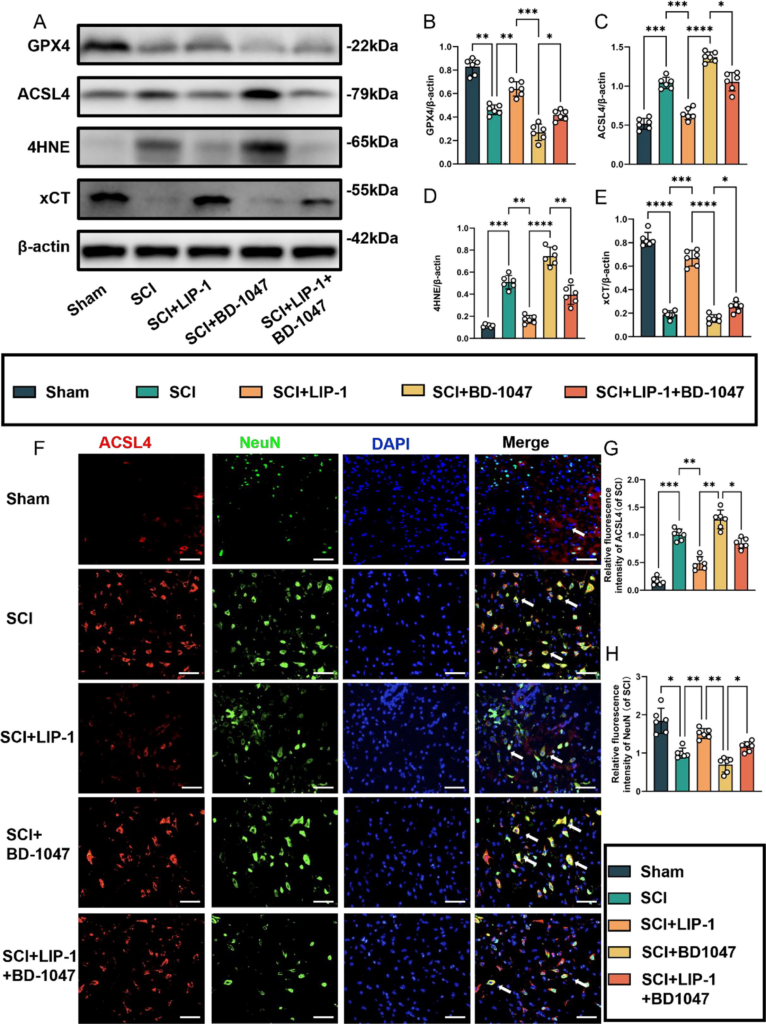
Activation of the Sigma-1 receptor can inhibit spinal cord ferroptosis.
(A) Western blotting was performed to assess the expression of GPX4, ACSL4, 4HNE, and xCT 7 days after SCI. (B-E) Quantitative analysis was conducted on the expression of GPX4, ACSL4, 4HNE, and xCT (n = 6, all data are expressed as mean ± standard deviation using one-way ANOVA). (F) Immunofluorescence staining was used to detect the levels of ACSL4 and NeuN in each group. (G, H) Statistical analysis was conducted on immunofluorescence staining of ACSL4-positive neuronal cells and neuronal cells in each group (of SCI group, n = 6, scale bar = 50 µm, all data are expressed as mean ± SD using one-way ANOVA). The results showed statistical significance with *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 by one-way ANOVA, n = 6 animals per group.
We investigated subsequently whether LIP-1 could alleviate ferroptosis exacerbated by Sig-1R inhibitors by performing immunofluorescence staining for ACSL4, NeuN, TUNEL, and FJC (Fig. 4F-H). Immunofluorescence staining revealed a significant reduction in ACSL4-positive neurones in the spinal cord tissue sections of the LIP-1 group compared with those of the SCI group (Fig. 4F, G). Additionally, ACSL4 activation was significantly reduced in the LIP-1 + BD-1047 group compared to the BD-1047 group (Fig. 4F–H). The trend for NeuN was the opposite of that of ACSL4.
Neuronal activity in the spinal cord tissue after SCI was detected by FJC (green) staining (Fig. 5A, B). Immunofluorescence staining further revealed a significant reduction in the number of FJC-positive cells in the LIP-1 group compared to the SCI group (Fig. 5B). Additionally, the number of FJC-positive cells was significantly lower in the LIP-1 + BD-1047 group than in the BD-1047 group (Fig. 5B). These results suggest that LIP-1 attenuates the aggravation of ferroptosis caused by the Sig-1R inhibitor BD-1047.
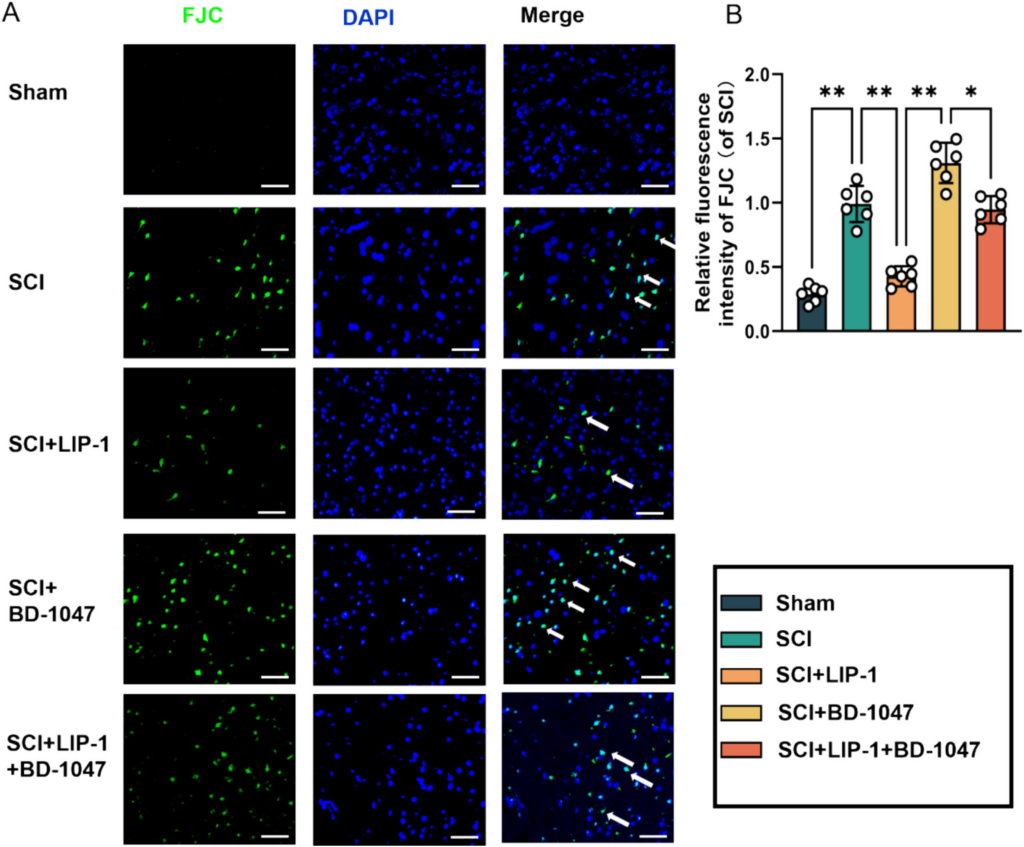
LIP-1 ameliorates Sigma-1 receptor inhibitor-enhanced neuronal apoptosis.
(A) Immunofluorescence staining detected representative images of FJC (green) staining in each group after seven days of spinal cord injury (SCI). Cell nuclei were stained with DAPI (blue) (scale bar = 50 µm). (B) The number of FJC-positive cells seven days after SCI was quantitatively analyzed(of SCI group, n = 6, scale bar = 50 µm, all data are expressed as mean ± SD using one-way ANOVA). The statistical analysis showed significant differences with *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 by one-way ANOVA, n = 6 animals per group. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.4. Sigma-1 receptor agonists attenuate IREα phosphorylation
Western blotting analysis revealed a significant increase in P-IREα expression in the SCI group (Fig. 6A–C). Protein expression was significantly decreased in SCI mice treated with FLV. Furthermore, the expression of these proteins increased in spinal cord-injured mice treated with BD-1047. In contrast, the expression of these proteins was reduced in the FLV + BD-1047 group. This group further shown an increase in P-IREα expression compared with the FLV group, but with no significant difference compared with the SCI group (Fig. 6D, E). The expression of these proteins increased, but not significantly, compared to that in the SCI group (Fig. 6D, E). Immunofluorescence staining further revealed a significant increase in P-IREα activation (red) and a decrease in NeuN levels (green) in the SCI group on spinal cord tissue sections (Fig. 6A-C). Compared to the SCI group, the FLV group showed a significant decrease in P-IREα activation (Fig. 6B). In contrast, the BD-1047 group showed a substantial increase in P-IREα activation (Fig. 6B). In the FLV + BD-1047 group, P-IREα activation was significantly increased compared to the FLV group. However, there was no significant difference compared to the SCI group (Fig. 6B). The trend of NeuN was opposite to that of P-IREα. Sig-1R agonists may attenuate IREα phosphorylation, reducing endoplasmic reticulum stress.
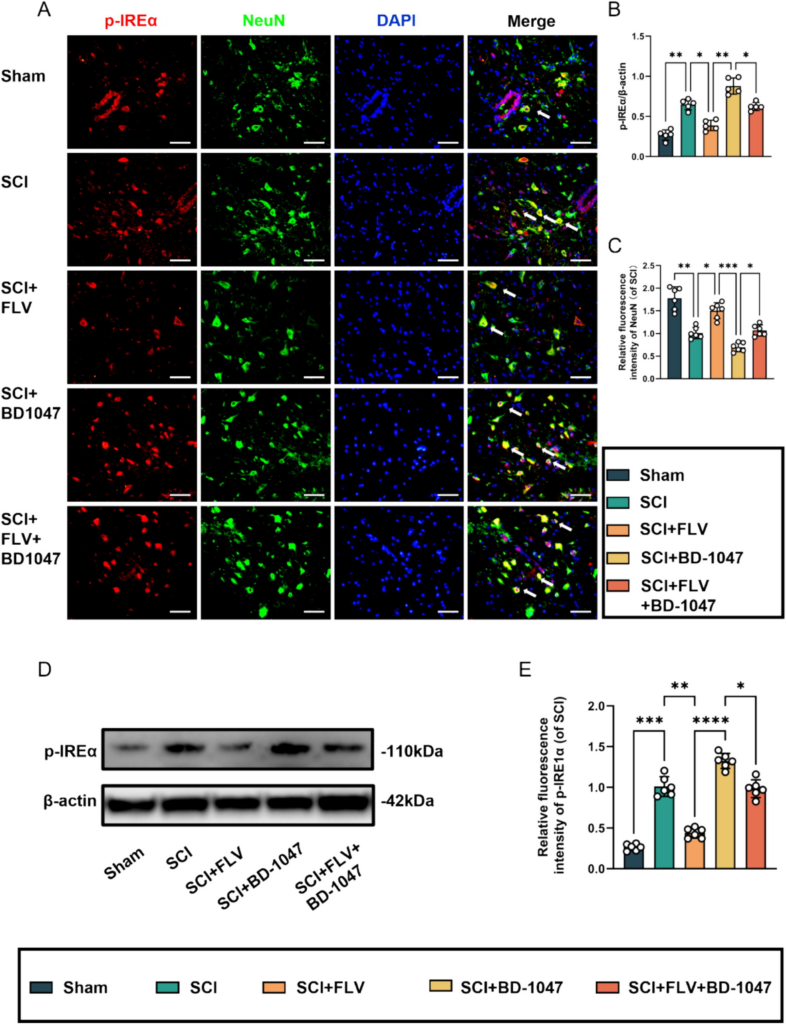
Sigma-1 receptor agonists attenuate IREα phosphorylation at seven days after SCI.
(A) Representative images of p-IREα (red) co-localized with neurones (NeuN, green) in each group after seven days of spinal cord injury (SCI) were detected by immunofluorescence staining. Cell nuclei were stained with DAPI (blue) (scale bar = 50 µm). (B-C) This study analyzed p-IREα-positive neuronal cells and immunofluorescence staining of neuronal cells in each group(of SCI group, n = 6, scale bar = 50 µm, all data are expressed as mean ± SD using one-way ANOVA). (D) Western blotting was performed to assess the expression of p-IREα 7 days after SCI. (E) Quantitative analysis was conducted on the expression of p-IREα (n = 6, all data are expressed as mean ± standard deviation using one-way ANOVA). The statistical analysis showed significant differences with *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 by one-way ANOVA, n = 6 animals per group. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.5. KIRA6 attenuates ferroptosis after SCI, and further ameliorates ferroptosis exacerbated by sigma-1 receptor inhibitors
In SCI mice treated with the IRE1α inhibitor KIRA6, the expression of GPX4 and xCT proteins significantly increased compared to the SCI group. Additionally, the expression of these proteins was significantly higher in the KIRA6 + BD-1047 group than that in the BD-1047 group (Fig. 7A, B, D). The expression levels of ACSL4 and 4HNE were lower in the KIRA6 group than in the SCI group. Conversely, the expression of these proteins in the injured spinal cord of mice increased after treatment with BD-1047 compared with that in the SCI group. The expression of these proteins was significantly lower in the KIRA6 + BD-1047 group than that in the BD-1047 group (Fig. 7A, C, and E). Neuronal activity and its relationship with ferroptosis were examined by immunofluorescence staining for ACSL4 and NeuN (Fig. 7F-H). Immunofluorescence staining further revealed a significant increase in ACSL4 activation (red) and a significant decrease in NeuN (green) in the SCI group on sections of spinal cord tissue (Fig. 7F-H). Compared to the SCI group, the KIRA6 group showed a significant decrease in ACSL4-positive neurones (Fig. 7F, G), whereas the KIRA6 + BD-1047 group showed a substantial increase in ACSL4-positive neurones compared to the BD-1047 group. The trend for NeuN was the opposite to that of ACSL4. This demonstrates that KIRA6 can reduce ferroptosis caused by Sig-1R inhibitors in spinal cord tissues.
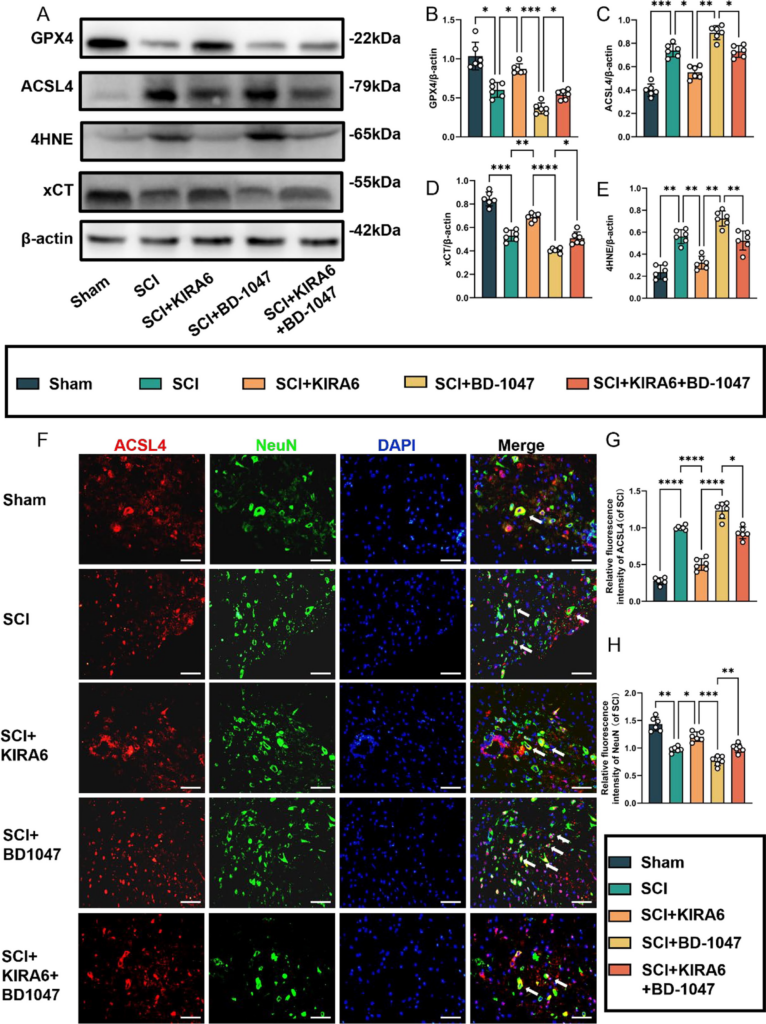
KIRA6 attenuates ferroptosis after SCI and also ameliorates ferroptosis exacerbated by sigma-1 receptor inhibitors
(A) Western blotting was performed to assess the expression of GPX4, ACSL4, 4HNE, and xCT 7 days after SCI. (B-E) Quantitative analysis was conducted on the expression of GPX4, ACSL4, 4HNE, and xCT (n = 6, all data are expressed as mean ± standard deviation using one-way ANOVA). (F) Immunofluorescence staining was used to detect the levels of ACSL4 and NeuN in each group. (G, H) Statistical analysis was conducted on immunofluorescence staining of ACSL4-positive neuronal cells and neuronal cells in each group (of SCI group, n = 6, scale bar = 50 µm, all data are expressed as mean ± SD using one-way ANOVA). The results showed statistical significance with *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 by one-way ANOVA, n = 6 animals per group.
4. Discussion
Ferroptosis is a regulated form of iron-dependent cell death characterised by the accumulation of lipid peroxidation to lethal levels (Lyamzaev et al., 2023, Stockwell, 2018). GPX4, ACSL4, 4HNE, and xCT are all essential markers of ferroptosis (Huang et al., 2022). In SCI, large amounts of oxidative stress products and inflammatory factors are produced at the site of injury. If these products are not cleared quickly, they can cause irreversible secondary damage to the spinal cord (Yu et al., 2023). Secondary damage usually occurs minutes to weeks after trauma, and involves various molecules and cells, including inflammatory and apoptotic factors (Anwar et al., 2016).
The sigma-1 receptor is a protein receptor widely distributed throughout the central nervous system (Su et al., 2016). It has a molecular chaperone activity, and has been implicated in the pathogenesis of various diseases. Recent studies have further shown that activation of Sig-1R can protect the nervous system from traumatic injury.
Similarly, other studies have demonstrated the correlation between ferroptosis and SCI. Therefore, we hypothesised that activating Sig-1R could protect the spinal cord tissue after SCI (Ma et al., 2023, Shen et al., 2024, Song et al., 2023). Ferroptosis induces stress in the endoplasmic reticulum of the CNS, further damaging it (Lee et al., 2018). In our experiments, we observed a significant improvement in the motor function of the lower limbs of SCI mice following the administration of a Sig-1R agonist. Previous studies have shown that Sig-1R agonists promote recovery of neurological function and reduce inflammation in mice with TBI (Hashimoto et al., 2024, Kaufmann et al., 2019, Sanchez-Blazquez et al., 2018).
Previous studies have also shown elevated levels of ferroptosis following SCI (Li et al., 2023). The key indicator GPX4 also showed a significant increase in SCI, indicating that ferroptosis plays a crucial role in this type of injury (Liu et al., 2024). To explore the protective role of Sig-1R activation in SCI mice, we administered Sig-1R agonists and inhibitors to the injured mice. We thus observed changes in ferroptosis-related markers, including GPX4, ACSL4, 4HNE, and xCT, in the spinal cord tissues. This study found that treating SCI mice with Sig-1R agonists significantly reduced ferroptosis levels in spinal cord tissues and ameliorated neuronal apoptosis. Conversely, the treatment of SCI mice with Sig-1R inhibitors resulted in a significant increase in ferroptosis. These results suggest that Sig-1R agonists protect the spinal cord by inhibiting ferroptosis. The immunofluorescence results for ACSL4 supported this conclusion.
LIP-1 is a classical ferroptosis inhibitor that effectively attenuates ferroptosis (Zhang et al., 2021). To investigate the correlation between Sig-1R and ferroptosis, we treated SCI mice with BD-1047, followed by treatment with the ferroptosis inhibitor LIP-1. Protein immunoblotting and immunofluorescence results revealed a significant reduction in ferroptosis levels in spinal cord neurons in the BD-1047 + LIP-1 group compared to those in the BD-1047 group after SCI. Therefore, it can be said that Sig-1R is closely associated with neuronal ferroptosis. Inhibition of Sig-1R expression significantly increased ferroptosis, which was ameliorated by treatment with a ferroptosis inhibitor. This finding is consistent with previous reports showing that Sig-1R attenuates the inflammatory response to traumatic injury (Shi et al., 2022). In addition, we observed that Sig-1R inhibitors promoted neuronal apoptosis and exacerbated spinal cord injury.
Previous articles have further shown that Sig-1R attenuates SCI by reducing the phosphorylation of IRE1α (Castany et al., 2018). However, no study has yet linked this phenomenon to ferroptosis. To demonstrate the link between Sig-1R and ferroptosis, we injected SCI mice with Sig-1R inhibitors and agonists. Protein immunoblotting and immunofluorescence showed that the amount of phosphorylated IRE1α was higher in the BD-1047 group than in the SCI group. Activation of IRE1α may further cause stress in the endoplasmic reticulum, aggravating the damage (Rainbolt and Frydman, 2020). Therefore, we concluded that Sig-1R affects ferroptosis in SCI through IRE1α, and plays a protective role in spinal cord tissues after SCI.
To confirm the influence of Sig-1R on ferroptosis in SCI through IRE1α, SCI mice were injected with an IRE1α inhibitor. The level of ferroptosis in SCI mice significantly improved after administration of the IRE1α inhibitor. However, this improvement disappeared after the administration of a Sig-1R receptor inhibitor. Therefore, it can be concluded that Sig-1R mediates ferroptosis through IRE1α. We hypothesised that the activation of Sig-1R would inhibit the phosphorylation of IRE1α, which protects neurones in spinal cord tissue.
It is unclear whether Sig-1R affects other signalling pathways in addition to being a widely present receptor in the central nervous system. Therefore, it remains necessary to determine whether Sig-1R plays a regulatory role in the ferroptosis signalling pathway. Recently, it was discovered that activation of Sig-1R alters microglial polarisation and has anti-inflammatory effects. Therefore, it is necessary to verify whether Sig-1R protects spinal cord tissues after SCI by exerting anti-inflammatory effects (Nepali et al., 2023). Overall, we demonstrated that Sig-1R activation ameliorates ferroptosis in spinal cord tissues, providing a reliable theoretical basis for treating spinal cord injury.
5. Conclusion
In summary, our findings suggest that activation of Sig-1R can reduce IREα levels, attenuating iron death after SCI and improving motor function. Thus, we showed that Sig-1R activation is a potential therapeutic strategy for treating patients with SCI impairments.