Sigma-2 receptor/TMEM97 agonist PB221 as an alternative drug for brain tumor
By Chia-Chi Liu, Ching-Fang Yu, Shu-Chi Wang, Hsueh-Yin Li, Chiu-Min Lin, Hsia-Han Wang, Carmen Abate, and Chi-Shiun Chiang
Excerpt from the article published in MC Cancer 19, 473 (2019). https://doi.org/10.1186/s12885-019-5700-7
Editor’s Highlights
- In adults, glioblastoma and astrocytoma are the most malignant and lethal forms of primary brain tumors.
- The antitumor activities of sigma-2 agonists are mediated mainly by mitochondrial superoxide production in both pancreatic and brain cancers.
- A non-lethal dose of sigma-2 agonist transiently inhibited the migration and invasion of ALTS1C1, which is similar to using a siRNA approach to silence the TMEM97 gene (proposed as a sigma-2 receptor gene); however, the effect of the latter is permanent, while the effect of a non-lethal dose of the ligand is transient.
Abstract
Background
There are limited effective drugs that can reach the brain to target brain tumors, in particular glioblastoma, which is one of the most difficult cancers to be cured from. Because the overexpression of the sigma-2 receptor is frequently reported in glioma clinical samples and associated with poor prognosis and malignancy, we herein studied the anti-tumor effect of the sigma-2 receptor agonist PB221 (4-cyclohexyl-1-[3-(5-methoxy-1,2,3,4-tetrahydronaphthalen-1-yl)propyl]piperidine) on an anaplastic astrocytoma tumor model based on previous encouraging results in pancreatic cancer and neuroblastoma SK-N-SH cells.
Methods
The expression of the sigma-2 receptor, transmembrane protein 97 (TMEM97), in ALTS1C1 and UN-KC6141 cell lines was measured by RT-PCR and quantitative RT-PCR. The binding of sigma-2 receptor fluorescent ligands PB385 (6-[5-[3-(4-cyclohexylpiperazin-1-yl)propyl]-5,6,7,8-tetrahydronaphthalen-5-yloxy]-N-(7-nitro-2,1,3-benzoxadiazol-4-yl)hexanamine) and NO1 (2-{6-[2-(3-(6,7-dimethoxy-3,4-dihydroisoquinolin-2(1H)-yl)propyl)-3,4-dihydroisoquinolin-1(2H)-one-5-yloxy]hexyl}-5-(dimethylamino)isoindoline-1,3-dione) was examined by flow cytometry and the fluorescent plate reader. The antitumor activity of PB221 was initially examined in the murine brain tumor cell line ALTS1C1 and then in the murine pancreatic cell line UN-KC6141. The potential therapeutic efficacy of PB221 for murine brain tumors was examined by in vitro migration and invasion assays and in vivo ectopic and orthotopic ALTS1C1 tumor models.
Results: The IC50 of PB221 for ALTS1C1 and UN-KC6141 cell lines was 10.61 ± 0.96 and 13.13 ± 1.15 μM, respectively. A low dose of PB221 (1 μM) significantly repressed the migration and invasion of ALTS1C1 cells, and a high dose of PB221 (20 μM) resulted in the apoptotic cell death of ALTS1C1 cells. These effects were reduced by the lipid antioxidant α-tocopherol, but not by the hydrophilic N-acetylcysteine, suggesting mitochondrial oxidative stress is involved. The in vivo study revealed that PB221 effectively retarded tumor growth to 36% of the control tumor volume in the ectopic intramuscular tumor model and increased the overall survival time by 20% (from 26 to 31 days) in the orthotopic intracerebral tumor model.
Background
In adults, glioblastoma and astrocytoma are the most malignant and lethal forms of primary brain tumors. The current standard of care for brain tumors is surgical removal of tumors as much as possible, followed by radiation therapy with concomitantly adjuvant chemotherapy with temozolomide (TMZ), as proposed by Stupp [1] several years ago. The prognosis still remains poor despite maximal efforts with this strict protocol [2,3,4]. The main therapeutic drug approved for glioma is TMZ, which targets mainly the guanine residues, susceptible to methylation, in DNA at the O6 position [5]. It is more effective against tumors lacking the O6-methylguanine DNA methyltransferase (MGMT). The extensive heterogeneity of brain tumors makes them resistant to the current single agent therapy and has strongly stimulated research into the development of multiple adjuvant therapies, including target therapy [6] or immunotherapy [7] and the invention of new drugs to target the specific pathways of brain tumor resistance [8].
The sigma receptor was initially described as an opioid receptor [9], but was later found to be distinct from the opioid receptors [10]. Two subtypes of this receptor have been found: sigma-1 and sigma-2 [11]. The sigma-1 protein was cloned in 1996 [12], but the sigma-2 receptor gene was not clearly identified until recently. A recent paper by Alon et al. [13] identified the sigma-2 receptor as transmembrane protein 97 (TMEM97), an endoplasmic reticulum (ER)-resident membrane protein also known as meningioma-associated protein (MAC30). Prior to the identification of the sigma-2 receptor as TMEM97, the function of the sigma-2 receptor was studied through high affinity ligands [11, 14,15,16,17] and appeared to be linked to neurodegenerative diseases [17] and cancer development [14]. Sigma-2 receptor ligands have not only been proposed as biomarkers for cancers, but also potential anticancer agents, because many of them could induce tumor cell death [18].
A high affinity sigma-2 receptor ligand, PB221 (4-cyclohexyl-1-[3-(5-methoxy-1,2,3,4-tetrahydronaphthalen-1-yl-propyl)] piperidine), derived from a class of N-cyclohexylpiperazine derivatives, has been recognized as a potent inhibitor of the proliferation of the SK-N-SH human neuroblastoma cell line in vitro [19]. Recent work on this compound further demonstrated that PB221 could induce apoptosis by generating superoxide radicals in mitochondria and activating caspase in several pancreatic cell lines in vitro, as well as retarding Panc02 tumor growth in C57BL/6 J mice [20].
The sigma-2 receptor (TMEM97) is highly expressed in human glioma tissues and its expression level is associated with the malignancy of glioblastoma [21]. The knockdown of the TMEM97 gene expression by specific siRNA could inhibit cell growth, migration, and invasion of glioma cell lines U87 and U373 [21]. This suggests that the sigma-2 receptor could be a potential target for brain tumor therapy. By using a pre-clinical astrocytoma model, the present study demonstrates that the high affinity sigma-2 receptor ligand PB221 could indeed delay the growth rate of intramuscular ALTS1C1 tumors and prolong the survival of mice bearing intracranial ALTS1C1 tumors. The thus results suggest that PB221 is a potential alternative therapeutic drug for treating brain tumors.
…
Results
The overexpression of the sigma-2 receptor in various types of cancers, including breast carcinoma, melanoma, prostate cancer, pancreatic tumors, and brain tumors [14, 27], has caused sigma-2 receptor ligands to be potential drugs for monitoring and treating cancer [18, 28]. Previous studies have shown that several derivatives of the high affinity sigma-2 receptor agonist PB28 (1-cyclohexyl-4-[3-(5-methoxy-1,2,3,4-tetrahydronaphthalen-1-yl)propyl]piperazine), have the ability to inhibit the growth of pancreatic cancer [20, 25] and the neuroblastoma SK-N-SH cell line [19]. Among the series of PB28 derivatives, PB221 (4-cyclohexyl-1-[3- (5-methoxy-1, 2, 3, 4-tetrahydronaphthalen-1-yl) propyl]peperidine) stood out for its sigma-2 vs. sigma-1 selectivity (sigma-1 Ki = 143 nM; sigma-2 Ki = 18.8 nM) and, more importantly, for its potent cytotoxic activity against neuroblastoma SK-N-SH cells (IC50 = 3.64 μM) [19]. The present study aimed to determine if PB221 also shows potential for treating brain cancer. Its cytotoxicity against the murine astrocytoma cell line ALTS1C1 and murine pancreatic cell line UN-KC6141 was initially examined by cytotoxicity assay. Figure 1a shows that the IC50 (half maximal inhibitory concentration) of PB221 for ALTS1C1 is 10.61 ± 0.96 μM (solid circle curve of Fig. 1a), which is significantly different from the IC50 of 11.78 ± 1.32 μM of its parental drug, PB28 (the ALTS1C1* open circle curve of Fig. 1a). The IC50 of PB221 for ALTS1C1 is also comparable with the IC50 of the murine pancreatic cell line UN-KC6141 of 13.13 ± 1.15 μM. The similar IC50 for these two cell lines correlated with the similar binding ability of these cells to the selective fluorescent sigma-2 receptor ligand, NO1 (Fig. 1b) [24]. This suggests that PB221 may have similar therapeutic potential for brain tumors as shown for pancreatic tumors through the binding of sigma-2 receptors [20].
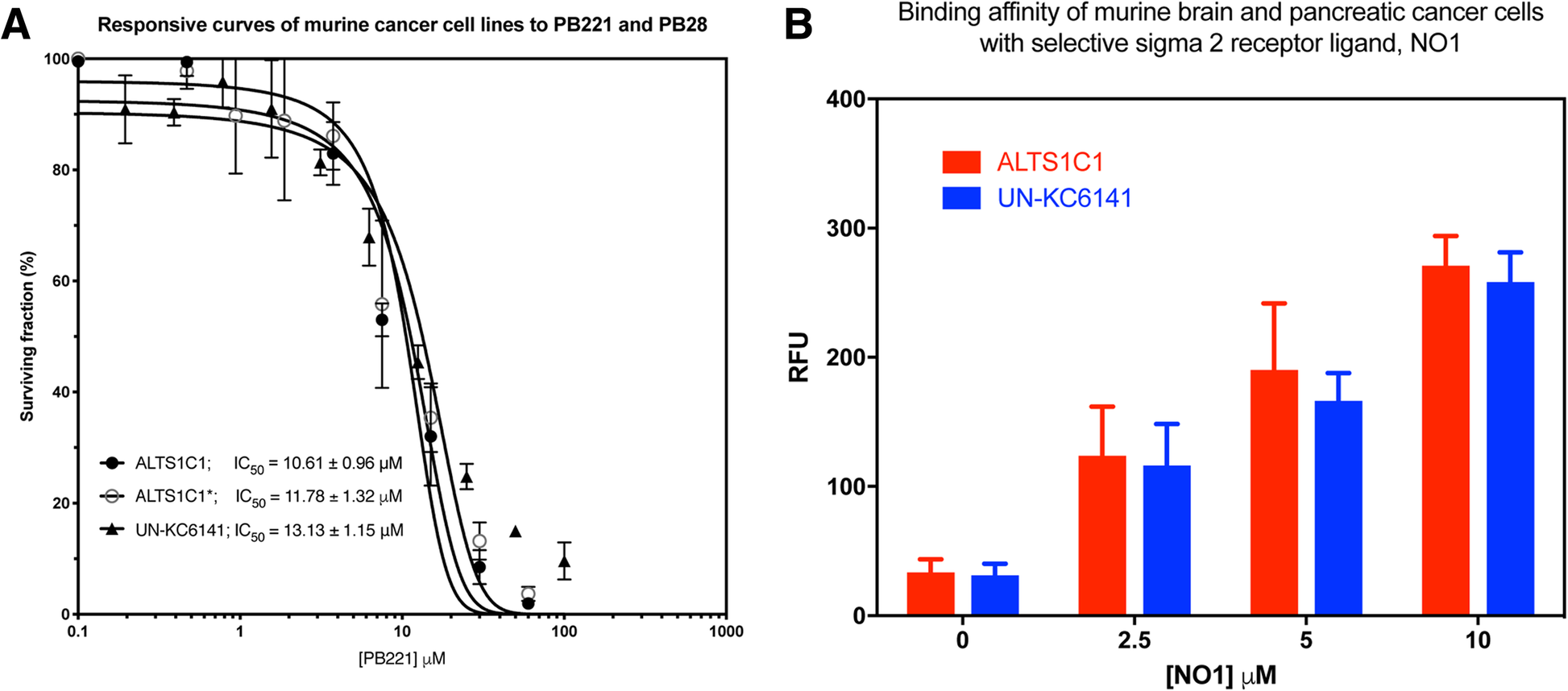
The responses of brain and pancreatic cell lines to the cytotoxicity of PB221 and binding affinity with selective sigma-2 receptor agonist ligand, NO1. (a) The survival curve of various cell lines in response to a range of PB221 and PB28 concentration for 3 days was measured by MTT assay. The IC50 for murine astrocytoma ALTS1C1 cells and murine pancreatic UN-KC6141 cells was 10.61 and 13.13 μM, respectively. ALTS1C1* open circle curve is the responsive curve of ALTS1C1 to PB28 and the IC50 is 11.78 μM. (b) The binding affinity of ALTS1C1 and UN-KC6141 cells with selective sigma-2 receptor ligand, NO1, was examined by fluorescent plate reader and the relative fluorescent unit (RFU) was measured with the excitation of 390 nm and emission at 525 nm
Additionally, RT-PCR (Fig. 2a) and quantitative RT-PCR (Fig. 2b) showed that both ALTS1C1 and UN-KC6141 cells expressed TMEM97 mRNA. TMEM97 was recently identified as the sigma-2 receptor [13] and is expressed by human glioma cell lines [21]. The suppression of TMEM97 could inhibit the proliferation, migration, and invasion of U87 and U373 human glioma cell lines [21]. The in vitro migration and invasion assays showed that a low dose (1 μM) of PB221, with minimal effects on the cell growth rate, significantly inhibited the migration (Fig. 3a and b, P < 0.05) and invasion (Fig. 3c and d, P < 0.001) of ALTS1C1 murine astrocytoma. The reduction of cell migration was significant at 16 h but was not very significant at 24 and 36 h, suggesting the inhibiting effect of the non-toxic dosage of PB221 on ALTS1C1 is transient. A recent publication shows that the activation of the sigma-2 receptor signaling pathway could lead to the production of mitochondrial superoxide in pancreatic cells [20]. Here, we found that the administration of PB221 could also increase the level of mitochondrial superoxide in murine astrocytoma cells (ALTS1C1) and human glioma cells (U87) (Fig. 4a). Furthermore, we found that the inhibitory effect of PB221 on the invasion, migration, and cell survival of ALTS1C1 was associated with mitochondrial superoxide production. Our results reveals that the effect of PB221-reduced migration and invasion (Fig. 4b and c) and the PB221-induced apoptotic cell death (Fig. 4d) on ALTS1C1 could be reduced by the lipid antioxidant α-tocopherol, but not by the hydrophilic N-acetylcysteine, as previously verified for PB28 derivatives [20] and by differently structured sigma-2 ligands, such as thiosemicarbazones in pancreatic tumor cells [29].
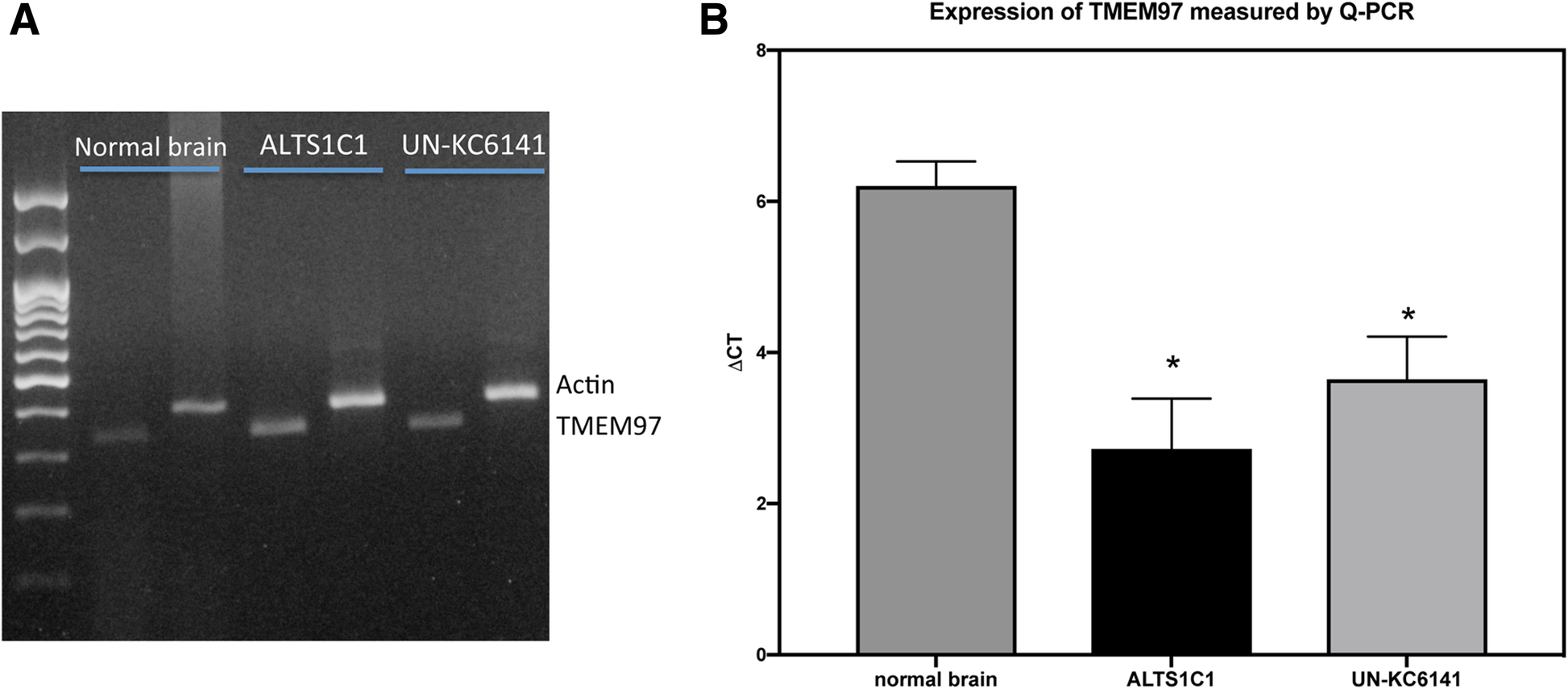
The expression of TMEM97 by ALTS1C1 and UN-KC6141 cells. The expression of TMEM97 mRNA by normal brain cells, ALTS1C1 and UN-KC6141 cells was assessed by (a) RT-PCR and (b) quantitative PCR (Q-PCR). The difference (ΔCt) between the Ct of the gene transcript and the endogenous control β-actin determined the gene expression level

Effects of PB221 on brain tumor cell migration and invasion. (a) Represented pictures of migration assay illustrate the retarded cell migration rate of ALTS1C1 cells following 1 μM PB221 treatment for 16 h. Scale bar = 100 μm. (b) A summary graph for the dose and time effects of PB221 on the migration distance of ALTS1C1 cells. *: P < 0.05 compared with control. (c) Represented pictures of invasion assay reveal the decrease of invasion cells following various doses of PB221 treatment for 16 h. (d) A summary graph for the dose effects of PB221 on ALTS1C1 cell invasion ability. ***: P < 0.001 compared with control
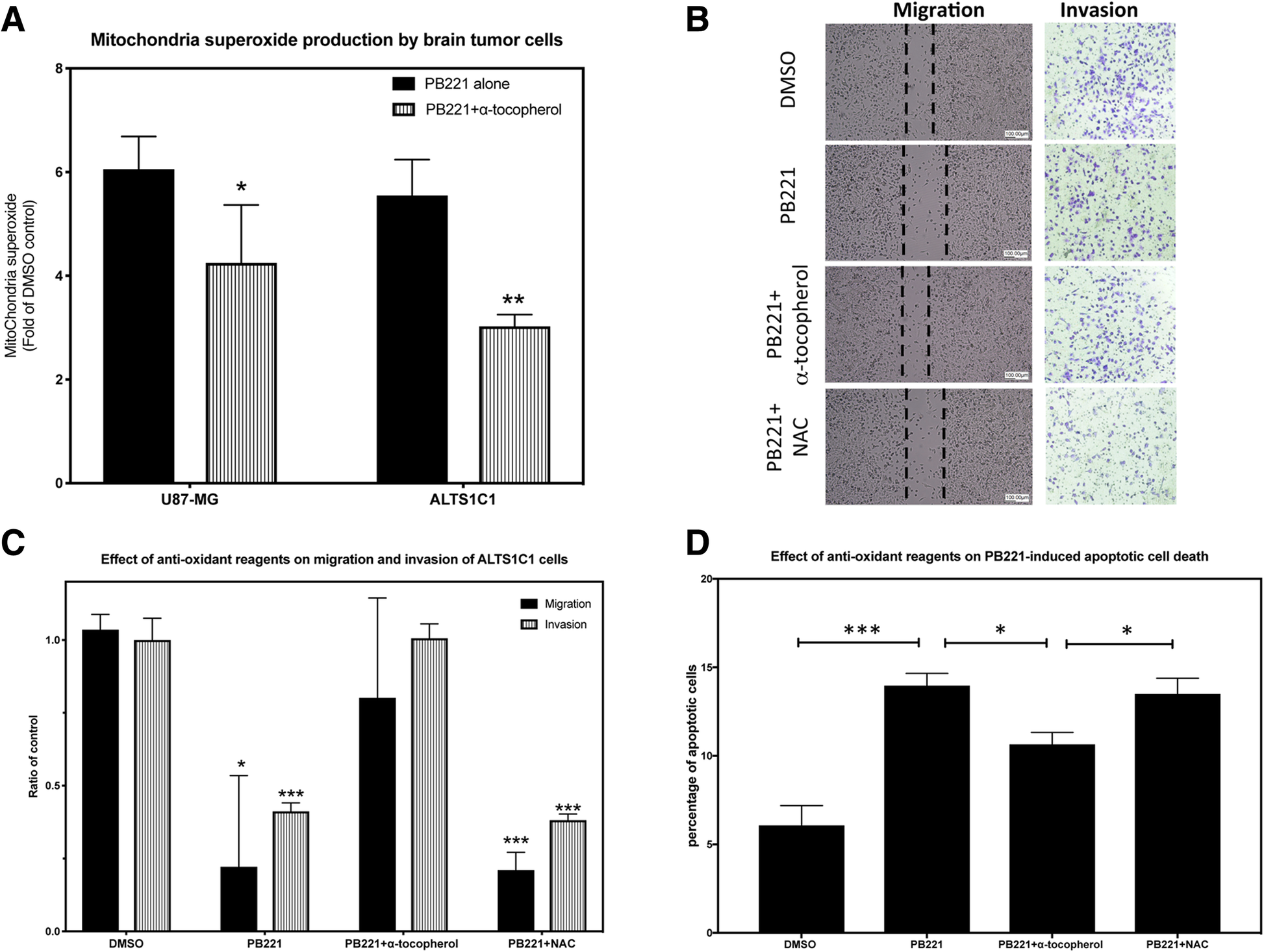
The effect of anti-oxidant on PB221 effects on ALTS1C1 (a) A summary graph reveals the level of mitochondria superoxide in brain tumor cell lines, U87-MG and ALTS1C1 following PB221 stimulation was measured by the fluorescent intensity of MitoSoxTM Red using flow cytometry. (b) Represented pictures of migration and invasion assays illustrate the effect of anti-oxidant α-tocopherol and NAC on PB221 (1 μM)-inhibited migration and invasion ability of ALTS1C1 cells. Scale bar = 100 μm. (c) A summary graph shows the preventing effect of 10 μM of α-tocopherol, but not NAC, on PB221 (1 μM)-inhibited migration and invasion ability of ALTS1C1 cells. (d) A summary graph reveals the preventing effect of 10 μM of α-tocopherol, but not NAC, on PB221 (20 μM)-induced apoptotic cell death of ALTS1C1 cells assayed by flow cytometry. *: P < 0.05. **: P < 0.01. ***: P < 0.001
The above in vitro results reveal the potential of PB221 as a target agent for sigma-2 overexpressing tumors. We used an intramuscular ALTS1C1 tumor model to further examine the potential of PB221 as a therapeutic drug for brain tumors. Figure 5a shows that the administration of four doses of PB221 (2 mg/mouse/injection) significantly (P < 0.001 at day 19) delayed the growth rate of ALTS1C1, with similar results as those for one dose of TMZ (2 mg/mouse/injection). However, the mice receiving one dose of TMZ were too sick to receive further treatment of TMZ. Conversely, mice could tolerate up to four doses of PB221, despite reduced body weight, which gradually returned to within the normal range in 11 days (Fig. 5b). The clinical importance of PB221 for brain tumors was further examined in an orthotopic model. Although the four doses of PB221 (2 mg/mouse/injection) was tolerable for mice carrying intramuscular tumors, the mice needed 10 days to regain their body weight. Despite this unwanted side effect, PB221 was not discarded as a potential therapeutic agent considering its promising effect against intracranial tumors. Rather, the administration protocol of PB221 was reduced to 1 mg/mouse/injection but extended to five doses in a week. Figure 5c shows that administering PB221 effectively prolonged the mean survival time of ALTS1C1 tumor-bearing mice by 20% (26 vs. 31 days, P < 0.0001). Figure 5d again demonstrates that five doses of PB221 (1 mg/mouse/injection) were tolerated well by treated mice bearing ALTS1C1 tumors for 10 days. Figure 5b and d also show that PB221 had similar side effects as the current therapeutic drug, TMZ, and was tolerable with the modified administration procedure. This result clearly demonstrates that PB221 has potential as an alternative agent for brain tumor therapy.
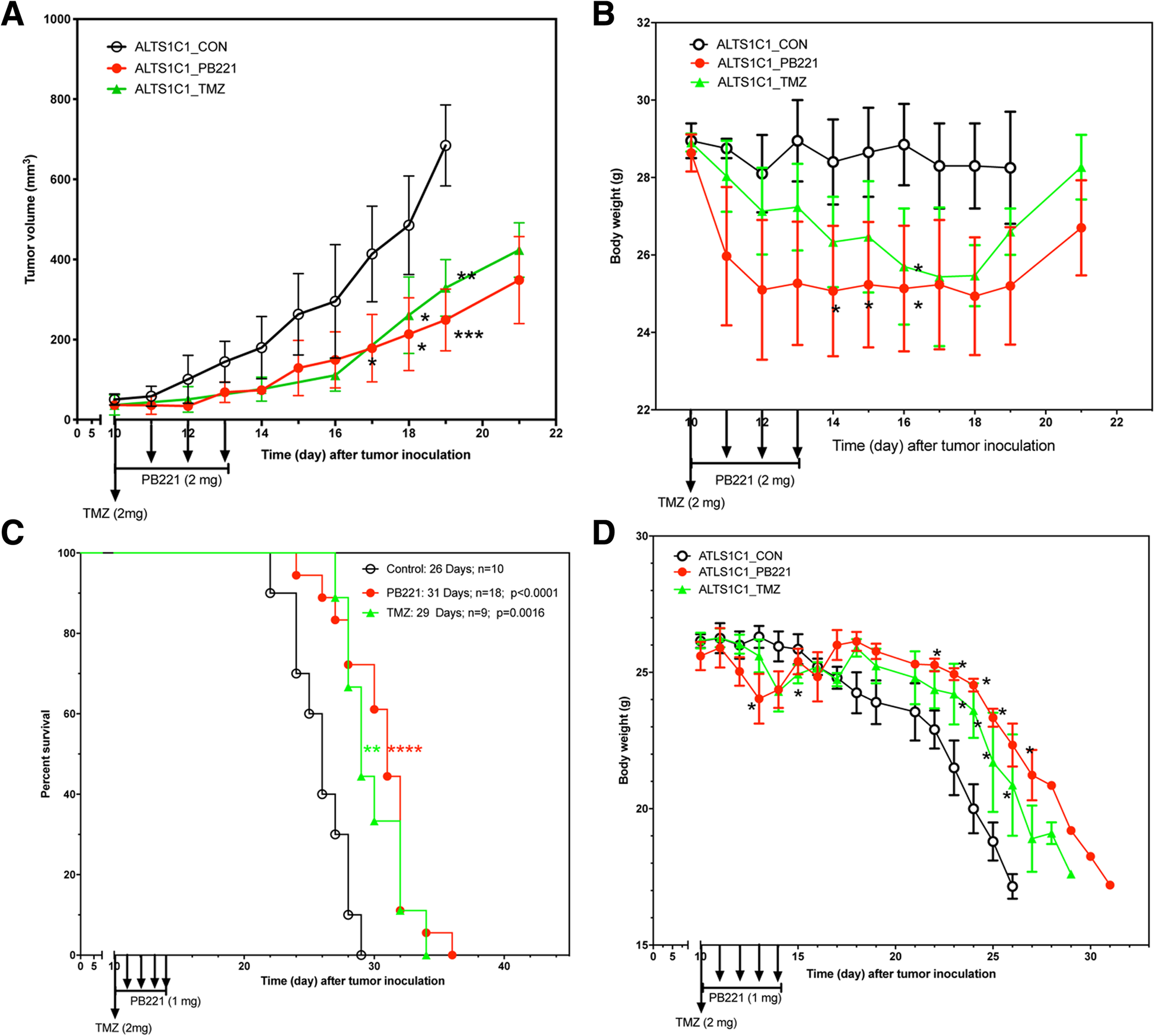
The in vivo anti-tumor effects of PB221 against ALTS1C1 tumors. (a) The tumor growth curves of intramuscular ALTS1C1 tumors following the treatment of PB221 and TMZ. One dose of TMZ (2 mg/mouse/injection) and 4 doses of PB221 (2 mg/mouse/injection) were intraperitoneally injected into tumor-bearing mice at day 10 following the tumor inoculation as indicated by the arrows in the graph. The data represented the average tumor volume in each group from one representative experiment of three independent experiments. *: P < 0.05. **: P < 0.01. ***: P < 0.001. (b) The change of mouse body weight following the intramuscular tumor implantation and the treatment of 4 doses of PB221 (2 mg/mouse/injection) and one dose of TMZ (2 mg/mouse/injection). *: P < 0.05. (c) The animal survival curve for mouse-bearing intracranial ALTS1C1 tumor and the treatment of 5 doses of PB221 (1 mg/mouse/injection) and one dose of TMZ (2 mg/mouse/injection). **: P < 0.01; ****: P < 0.0001. (d) The change of mouse body weight following the intracranial tumor inoculation and the treatment of 5 doses of PB221 (1 mg/mouse/injection) and one dose of TMZ (2 mg/mouse/injection). *: P < 0.05. **: P < 0.01
Discussion
After more than a decade of trials with various novel compounds, the poor prognosis for glioma patients is still a challenge for brain tumor therapy. TMZ-based radiotherapy remains the standard care and main treatment strategy despite the static survival rate of around 15%. Also, the 20% increase in the median survival rate from radiotherapy (12.1 to 14.6 months) by TMZ [1] remains unchanged because TMZ mainly targets tumors with aberrant MGMT proteins. Finding new compounds is urgently needed. In this study, we reported the potential of the sigma-2 receptor agonist PB221 for treating brain tumors in a murine ALTS1C1 tumor model. We demonstrated that the tolerated dose of PB221 could increase the survival time of mice bearing intracranial brain tumors by 20%.
The overexpression of the sigma-2 receptor by cancer cells has been reported in several types of tumors, including breast, pancreatic, and brain tumors [14, 18]. This has led to the discovery of sigma-2 receptor ligands as potential compounds in cancer diagnosis and therapy [28]. Among the many sigma-2 receptor ligands, serial analogues of PB28 have been developed and their potential for cancer imaging and therapy, addressed [15, 16, 18,19,20, 24, 25, 30]. Berardi et al. showed that one PB28 derivative, PB221, had a 3-fold increase on in vitro antiproliferation activity against neuroblastoma SK-N-SH cells compared with parental PB28 (EC50 = 3.64 ± 0.68 vs 9.97 ± 0.55) [19]. The structural difference in the basic moiety (piperidine for PB221 and piperazine for PB28) of the two compounds causes PB221 to have a much higher selectivity for the sigma-2 receptor than PB28. Furthermore, a recent study by Pati et al. not only demonstrated PB221 as a promising drug for pancreatic cancer, but also identified the cytotoxic pathway of PB221 which involves elevating mitochondrial superoxide production and causing caspase activation in Panc02 pancreatic cancer cells [20]. Our study demonstrated that PB221 not only has antiproliferation activity against brain tumor cells, but also retards the migration and invasion ability of highly invasive murine astrocytoma cells in vitro.
Previous studies have shown that different structures of PB28-derived sigma-2 ligands killed pancreatic tumor cells through different mechanisms. For example, the killing action of PB183 [20] and SW43 [31] of pancreatic cancer cells is caspase-independent and relies more on cellular oxidation. Conversely, the antitumor effect of PB221 depends on the caspase-associated apoptotic pathway and mitochondrial superoxide activity [20, 29]. We found that the increase in the level of mitochondrial superoxide caused by the administration of PB221 also occurred in murine astrocytoma cells (ALTS1C1) and human glioma cells (U87). This suggests that an oxidation-associated pathway may similarly be involved in the cytotoxic effect of PB221 on brain tumor cells with PB221-induced apoptotic cell death counteracted by the lipid antioxidant α-tocopherol, but not by the hydrophilic N-acetylcysteine. Indeed, this study illustrated that the effects of PB221 on slowing brain tumor cell migration and invasion was through mitochondrial superoxide production. This evidence demonstrates that the antitumor activities of PB221 are mediated mainly by mitochondrial superoxide production in both pancreatic [20, 29] and brain (current study) cancers. These results are in agreement with similar results from thiosemicarbazones sigma-2 ligands in pancreatic tumor cells [29], confirming the mitochondrial superoxide production as a common outcome of sigma-2 receptor ligand activity. Our results showed that a non-lethal dose of PB221 transiently inhibited the migration and invasion of ALTS1C1, which is similar to using an siRNA approach to silence the TMEM97 gene (proposed as a sigma-2 receptor gene) [13]; however, the effect of the latter is permanent, while the effect of a non-lethal dose of PB221 is transient. This, together with previous biological data on binding and activity in sigma-2 overexpressing cells [19, 20], supports the idea that the sigma-2 receptor is a likely target molecule of PB221. In addition, our result also showed that PB221 could inhibit the proliferation of murine astrocytoma cells (ALTS1C1) and murine pancreatic cells (UN-KC6141). All of these cells not only express mRNA of the TMEM97 gene, but also strongly bind to the sigma-2-selective fluorescent tracer (NO1) [24]. This further supports PB221 as a selective agonist of the sigma-2 receptor. Finally, ALTS1C1 cells also showed a good binding affinity to another selective sigma-2 receptor ligand, PB385 (Additional file 1: Figure S1). However, besides the sigma-2 receptor, other targets for PB221 should not be ruled out.
Conclusions
This study illustrated that a tolerable dose of PB221 could delay tumor growth and extend the survival time of ALTS1C1 tumor-bearing mice by 20%, which is slightly better than a similar tolerable dose of TMZ (~ 12% increase of survival time). These results propose PB221 as a potential drug for treating brain tumors.

