Role of the Ca2+ channel α2δ-1 auxiliary subunit in proliferation and migration of human glioblastoma cells
By Miriam Fernández-Gallardo, Alejandra Corzo-Lopez, David Muñoz-Herrera, Margarita Leyva-Leyva, Ricardo González-Ramírez, Alejandro Sandoval, Rodolfo Delgado-Lezama, Eduardo Monjaraz, and Ricardo Felix
Excerpt from the article published in PLoS ONE 17(12): e0279186, December 15, 2022, DOI: https://doi.org/10.1371/journal.pone.0279186
Editor’s Highlights
- Glioblastoma multiforme (GBM) is the most aggressive primary brain tumor, accounting for >70% of all brain tumors in adults over 50 years of age.
- The alpha2delta-1 (α2δ-1) subunit is a protein with complex topological features.
- Its significant role in tumor progression has already been demonstrated previously in several types of cancer cell lines, including larynx, lung, ovary, liver, stomach, and breast
- The α2δ-1 subunit cellular actions may be grouped into two general mechanisms. The first of them would be directly associated with the function of the subunit as part of the high-threshold Ca2+ channel complex, while the second would be independent of its role in the channels.
- Findings strongly suggest that the activation of TLR-4 increases the expression of α2δ-1 in U87 cells, favoring their proliferative and migratory potential, which might eventually provide a theoretical basis to examine novel treatment of GBM.
Abstract
The overexpression of α2δ-1 is related to the development and degree of malignancy of diverse types of cancer. This protein is an auxiliary subunit of voltage-gated Ca2+ (CaV) channels, whose expression favors the trafficking of the main pore-forming subunit of the channel complex (α1) to the plasma membrane, thereby generating an increase in Ca2+ entry. Interestingly, TLR-4, a protein belonging to the family of toll-like receptors that participate in the inflammatory response and the transcription factor Sp1, have been linked to the progression of glioblastoma multiforme (GBM). Therefore, this report aimed to evaluate the role of the α2δ-1 subunit in the progression of GBM and investigate whether Sp1 regulates its expression after the activation of TLR-4. To this end, the expression of α2δ-1, TLR-4, and Sp1 was assessed in the U87 human glioblastoma cell line, and proliferation and migration assays were conducted using different agonists and antagonists. The actions of α2δ-1 were also investigated using overexpression and knockdown strategies. Initial luciferase assays and Western blot analyses showed that the activation of TLR-4 favors the transcription and expression of α2δ-1, which promoted the proliferation and migration of the U87 cells. Consistent with this, overexpression of α2δ-1, Sp1, and TLR-4 increased cell proliferation and migration, while their knockdown with specific siRNAs abrogated these actions. Our data also suggest that TLR-4-mediated regulation of α2δ-1 expression occurs through the NF-kB signaling pathway. Together, these findings strongly suggest that the activation of TLR-4 increases the expression of α2δ-1 in U87 cells, favoring their proliferative and migratory potential, which might eventually provide a theoretical basis to examine novel biomarkers and molecular targets for the diagnosis and treatment of GBM.
Introduction
Glioblastoma multiforme (GBM) is the most aggressive primary brain tumor, accounting for >70% of all brain tumors in adults over 50 years of age. Despite the various treatments for GBM (surgery, radio, and chemotherapy), it remains a disease with a poor prognosis for survival [1, 2]. Indeed, despite the development in research on different molecular aspects of GBM, the tumor continues to have one of the highest mortality rates; consequently, there is a growing interest in learning more regarding its origin and development.
On the other hand, Ca2+ is a ubiquitous divalent cation and a second messenger that participates in multiple physiological processes regulated by transient changes in its intracellular concentration. Therefore, intracellular calcium concentrations are determined by an elaborate system of transporters, channels, membrane exchangers, binding/buffer proteins, and ATPases that finely regulate the flow of this cation in and out of cells and between different cellular organelles [3, 4]. When an alteration occurs in these systems, changes are caused in the dynamics of Ca2+ flows, affecting the functioning of various cellular events such as proliferation, gene expression, protein phosphorylation/dephosphorylation, and cell death [4–6].
In this context, voltage-gated Ca2+ (CaV) channels have been shown to play a fundamental role in tumor biology. Specifically, these channels are known to exhibit altered functional expression or aberrant localization associated with the promotion of tumor growth and cell migration, as well as resistance to treatment [4–8]. CaV channels are transmembrane oligomeric complexes conformed by an ion-conducting α1 subunit and auxiliary α2δ and β subunits that are activated by cell membrane depolarization. CaV channels conduct Ca2+ into cells, where they initiate a wide variety of physiological responses, ranging from hormone and neurotransmitter release and muscle contraction to gene transcription, among many others [9]. In addition, these channels often associate with other proteins creating micro or nanodomains of Ca2+ signaling [10–12].
It has been previously reported also that the overexpression of the protein α2δ-1 is related to the development and degree of malignancy of numerous types of cancer, such as larynx, lung, ovary, liver, stomach, and breast [13–20]. In addition, it is known that the promoter region of the CACNA2D1 gene, which encodes for α2δ-1, has two functional Sp1 binding sites [21, 22]. Interestingly, various reports have shown the over-expression of Sp1 in diverse types of cancer, relating its expression level to tumor stage and unfavorable prognosis for patient survival [23–25]. Likewise, the silencing or inhibition of Sp1 reduces tumor formation, growth, and metastasis [26]. Interestingly, it has been reported that the expression of Sp1 in tumor cells induces the expression of diverse growth factors related to tumor growth and metastasis [27]. Hence, Küper et al. (2012), using renal medullary collecting duct cells, found that activation of the toll-like receptor TLR-4 promoted the activity of the transcription factor NF-кB, which participates in the regulation of Sp1 expression [28].
Based on these findings, in the present report, we evaluated the contribution of α2δ-1 to the proliferation and migration processes of U87 human glioblastoma cells and its possible regulation through the activation of TLR-4 and Sp1 signaling pathway. Our results support the idea that the activation of TLR-4 triggers the nuclear translocation of the transcription factor NF-кB, with the consequent increase in Sp1 expression, which regulates the presentation of α2δ-1.
…
Results
Expression of α2δ-1, TLR-4–1, and Sp1 in the U87 human glioblastoma cell line
To study the role of the CaV channel α2δ-1 subunit in the proliferative and migratory capacity of U87 cells, and whether the regulation of its expression was dependent on Sp1 after activation of TLR-4, we initially investigated the expression of these proteins in the cell line by Western blot, using antibodies available from commercial sources (see Material and Methods Section) and the human neuroblastoma SHSY-5Y cells as a positive control. Previous reports have documented the expression of α2δ-1, TLR-4, and Sp1 in the SHSY-5Y cell line [21, 29]. The results of the Western blot analysis showed the presence of strong bands at the predicted molecular weight for α2δ-1, TLR-4, and Sp1 (~150, ~95, and ~90 kDa, respectively) in the lysates of the two cell lines studied (S1 Fig), corroborating the expression of these three proteins in U87 human glioblastoma cells.
TLR-4 activation promotes the expression of α2δ-1
We next evaluated whether the activation of TLR-4 could regulate the transcription and expression of α2δ-1. To this end, luciferase assays were conducted to assess the activity of the α2δ-1 promoter in the presence and absence of the TLR-4 activator, LPS. The U87 cells were transfected with an expression vector containing the α2δ-1 promoter and luciferase as a reporter gene, and the cells were treated with LPS for 48 h to evaluate the promoter activity. The results showed that the activation of TLR-4 with LPS (5 μg/mL) significantly increased luciferase activity compared to the control (Fig 1A). In an additional series of experiments, the effect of the LPS treatment on the expression of the α2δ-1 protein was evaluated. Fig 1B shows a representative image of a semiquantitative Western blot experiment, where a higher density band is observed in the lysates from U87 cells treated with LPS compared to the loading control (β-actin). Last, Fig 1C shows that, on average, the level of α2δ-1 protein expression was ∼1.8-fold higher in LPS-treated U87 human glioblastoma cells compared to untreated controls. These data suggest that the activation of TLR-4 by LPS may trigger signaling pathways that positively regulate the expression of α2δ-1.
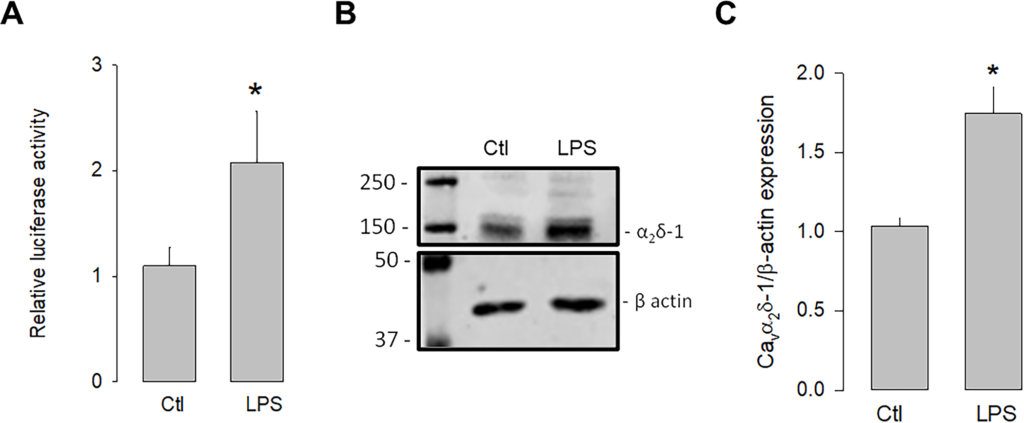
Upregulation of α2δ-1 by LPS.
U87 cells were co-transfected with a vector containing the α2δ-1 subunit promoter and the Renilla luciferase coding sequence (as a reporter gene), and the pRSV-βGal vector, which included the RSV promoter and the β-gal coding sequence to correct for differences related to transfection efficiency. Cells were incubated for 48 h in the absence and presence of the TLR-4 activator, LPS. B) Western blot assay performed on U87 cell lysates in the absence and presence of LPS. The image shows a representative assay of three performed separately. C) Densitometric analysis of protein α2δ-1 normalized with respect to the expression of β actin and depicted as a fold change from the control. Asterisks denote statistically significant differences (P < 0.05) with respect to the control (Ctl).
https://doi.org/10.1371/journal.pone.0279186.g001
TLR-4 activation favors U84 cells proliferation and migration
To study the effects of the TLR-4 activation with LPS on the proliferative capacity, U87 cells were counted automatically after 48 h of incubation in the absence and presence of the lipopolysaccharide. Fig 2A shows that the receptor activation caused a significant increase (>3-fold) in the proliferative capacity of U87 cells compared to the controls. Likewise, to corroborate that the effect produced by the treatment with LPS was the result of the activation of TLR-4, a specific antagonist of the receptor called C34 was used, and the results showed that in the presence of C34, the effect of LPS on cell proliferation were significantly prevented (Fig 2A).
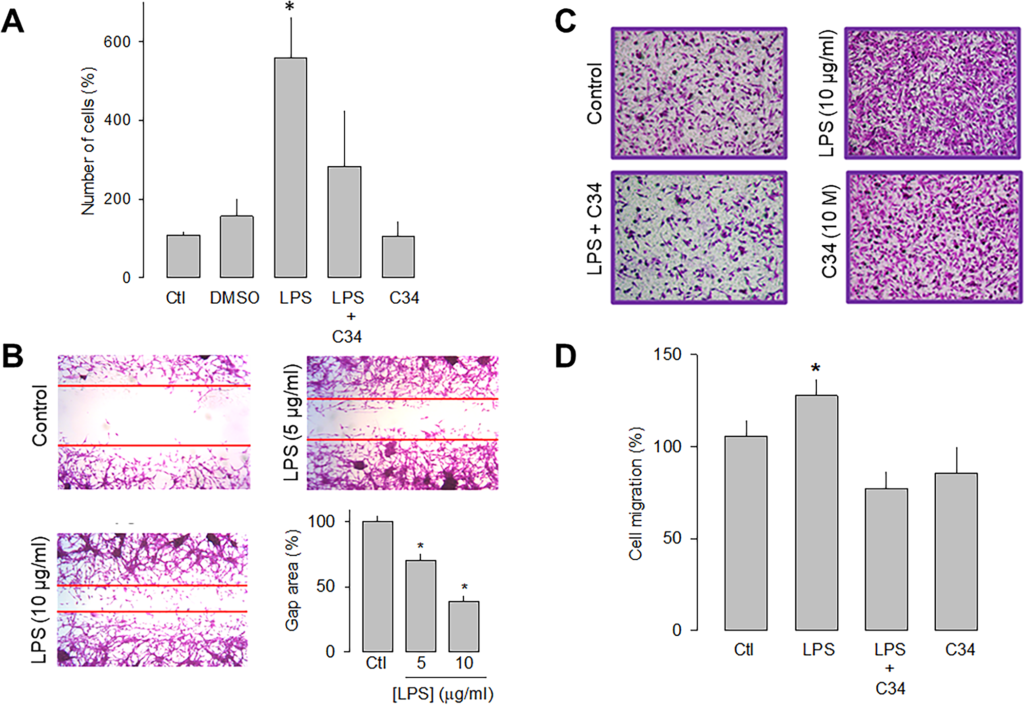
Effect of LPS on the proliferation and migration of U87 cells.
A) Comparison of direct and automated cell counting of U87 cells maintained 48 h in culture in the presence and absence of the TLR-4 activator, LPS, as indicated. Cell counts were performed in parallel after treatment with C34, a specific inhibitor of TLR-4, and the vehicle (DMSO) in which it was dissolved. Values are expressed as a percent of control, and each bar represents the mean ± SE of triplicate determinations in 3 separate experiments. B) Cell wound healing assays showing the ability of U87 cells to migrate in culture in the absence and presence of LPS, as indicated. The lower right panel shows a graph of the quantification of the wound area. C) Transwell migration assays of U87 cells in the presence of the activator (LPS) and the inhibitor (C34) of TLR-4, as listed. Typical images from three separate experiments are shown. D, Comparative analysis of transwell migration assay results of U78 cells as in C. Results represent the mean ± SEM of 3 independent experiments. *P < 0.05 compared to untreated cells.
https://doi.org/10.1371/journal.pone.0279186.g002
The effects of LPS on the migration of the U87 human glioblastoma cells were then evaluated semiquantitatively using wound healing and migration assays in transwell chambers. After LPS treatment (48 h), the cell monolayer was wounded, and 12 h later, the culture dishes were fixed and stained for analysis. The results show that in the presence of 5 μg/mL of LPS, the wound area was significantly smaller than the controls. This is even more evident at a higher concentration of the lipopolysaccharide (10 μg/mL; Fig 2B).
On the other hand, the effect of LPS on cell migration was studied, using the antagonist C34 to verify that the activation of TLR-4 was indeed involved in this effect. Representative images of four independent experiments under the different experimental conditions (control, LPS, LPS+C34, and C34 alone) are shown in Fig 2C, and the summary of the results is shown in Fig 2D. This data confirmed that U87 glioblastoma cell migration is favored in the presence of LPS. Interestingly, as occurred in the wound healing assay, the presence of the TLR-4 antagonist prevents the stimulatory effect of LPS. Together, these data show that the activation of TLR-4 may promote the migration process of U87 cells.
The overexpression of α2δ-1 increases cell proliferation and migration
Once the effect of LPS on the proliferation of U87 cells was evaluated, we next sought to define the effect of the α2δ-1 CaV channel auxiliary subunit overexpression on cell proliferation. Initial experiments showed that 1 μg/μL was determined to be the optimal concentration for plasmid transfection and was used in subsequent experiments. The upper panel in Fig 3A shows a representative Western blot image of the overexpression of α2δ-1, while the bottom panel summarizes the results of three separate experiments. These results corroborate the expression of the α2δ-1 subunit in the U87 cells and show a significant increase in the protein after transfection with the plasmid encoding the CaV channel auxiliary subunit.
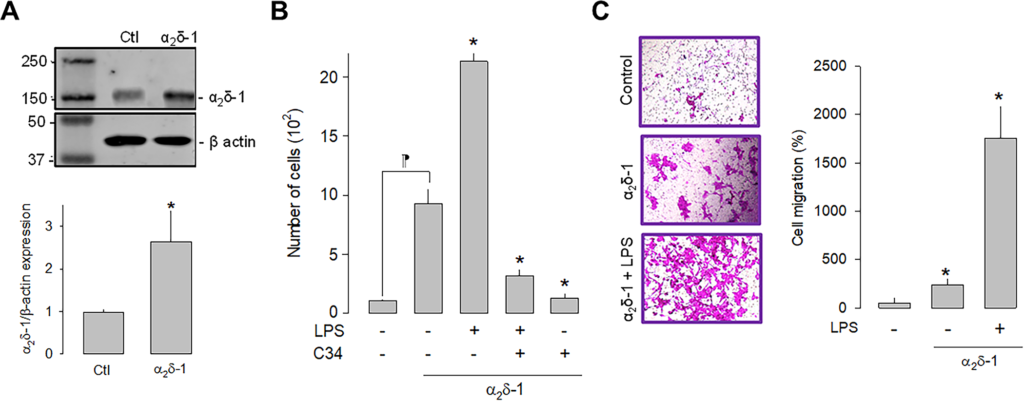
Effect of α2δ-1 overexpression on the proliferation and migration of U87 cells.
A) Western blot analysis of U87 cell lysates in control and α2δ-1 transiently transfected cells. The image in the upper panel shows a representative assay of three performed separately. The lower panel shows the densitometric analysis of the protein α2δ-1 normalized with respect to the expression of β actin and depicted as a fold change from the control. Asterisks denote a statistically significant difference (P < 0.05) with respect to the control (Ctl). B) Comparison of direct and automated cell counting of U87 cells maintained in culture after α2δ-1 transfection in the presence and absence of the agonist and the antagonist of TLR-4, LPS, and C34, respectively. Values are expressed as the number of cells in each experimental condition, and each bar represents the mean ± SEM of triplicate determinations in 3 separate experiments. *P < 0.05 versus α2δ-1 overexpressing cells (second bar from the left). ⁋P < 0.05 versus untransfected cells. C) Transwell migration assays of U87 cells in the presence of the TLR-4 activator (LPS). The left panel shows representative images from three separate experiments. The right panel shows the comparative analysis of the mean ± SEM of 3 transwell independent experiments. *P < 0.01 compared to untreated cells.
https://doi.org/10.1371/journal.pone.0279186.g003
We next investigated whether the activation or the inhibition of TLR-4, by LPS or C-34, respectively, were affected by α2δ-1 overexpression. This analysis showed that the overexpression of the CaV channel subunit per se significantly increases the number of cells and that this effect is more evident in the presence of LPS. As expected, the effect of LPS is prevented when C34 is present (Fig 3B). These findings suggest that α2δ-1 favors cell proliferation via activation of TLR-4 in U87 glioblastoma cells. Similarly, cell migration was favored after overexpression of α2δ-1. The results of the cell migration assays in transwell chambers under control conditions and after overexpression (in the presence and absence of LPS) showed a significantly higher number of cells when α2δ-1 was overexpressed (Fig 3C).
Inhibition of α2δ-1 prevents the effect of LPS on cell migration
Previous studies have shown that the α2δ-1 subunit increases the cell membrane expression of native and recombinant CaV channels. Likewise, it is also known that this protein makes the channels sensitive to the inhibitory effects of a group of antiepileptic/analgesic drugs called gabapentinoids, which includes gabapentin, pregabalin, and AdGABA [30, 31]. Given that the data in the preceding section implicate a role of α2δ-1 in cell migration, we next sought to explore whether gabapentin was also able to affect the migration of U87 cells. The results of this analysis showed that chronic exposure (48 h) to gabapentin (25 μM) prevented the stimulatory effect of LPS on the migratory capacity of the neuroblastoma U87 cells and corroborated the contribution of α2δ-1 to the cell migration process (Fig 4).
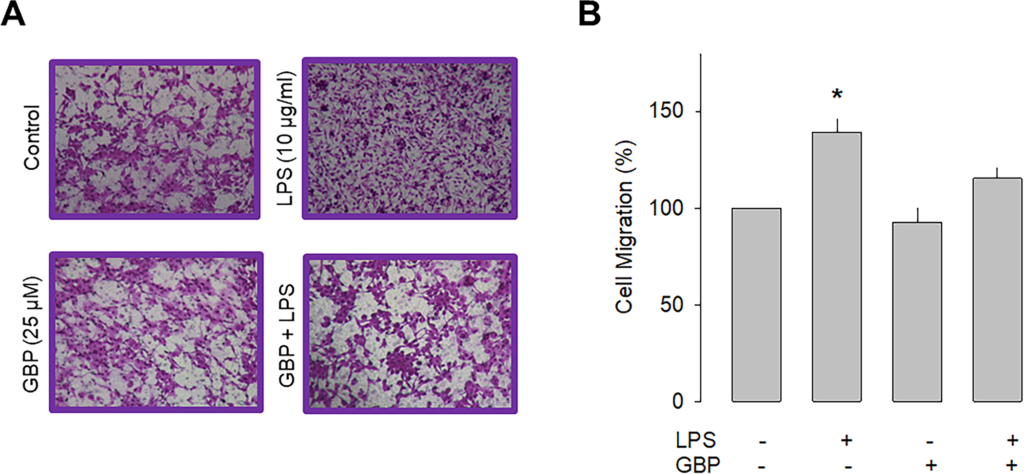
Effect of gabapentin on U87 cell migration.
A) Representative images from three separate experiments of transwell migration assays in the presence or absence of the TLR-4 activator (LPS) and the antagonist of the α2δ-1 subunit (gabapentin). B) Comparative analysis of the mean ± SEM of 3 transwell independent experiments. *P < 0.05 compared to untreated cells.
https://doi.org/10.1371/journal.pone.0279186.g004
Thrombospondin/α2δ-1 interaction increase cell proliferation and migration
The experimental evidence suggests a direct action of LPS stimulation on α2δ-1-mediated cell proliferation and migration. To further narrow down the specificity of α2δ-1 involvement in these cellular events, we performed a series of experiments in the presence of thrombospondin-1, an endogenous ligand of the α2δ-1 subunit [32]. Since thrombospondins are extracellular matrix molecules relevant in synaptogenesis and other cellular organization events [33], such experiments could hint at possible specific interactions between gliomablastoma cells and their cellular environment. Thus, we then determined the ability of thrombospondin to induce proliferation in U87 cells. After 48 h of stimulation, the dose-response curve presented in Fig 5Ashows that TSP induces a significant increase in the proliferation of U87 cells, with half of the maximum attained at ~0.5 nM and a maximum effect observed at 1 nM. Stimulation by TSP at 1 nM increased cell proliferation at a similar level to that induced by LPS (5 μg/ml). In subsequent experiments, the effect of TSP on the migration of U87 cells was examined (Fig 5B). Hence, this analysis shows that incubation with TPS also significantly affects cell migration, producing an increase of ~25% respect to the control condition (Fig 5C). These data support the idea that the α2δ-1 subunit regulates proliferation and migration in U87 cells.
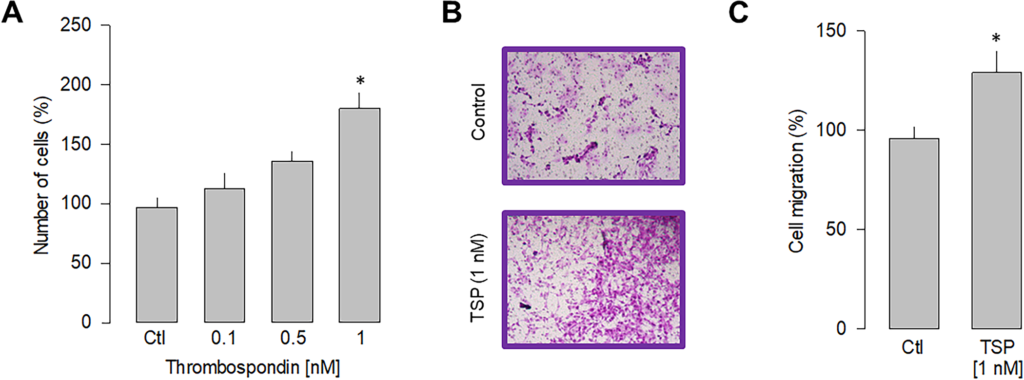
Effect of thrombospondin-1 on proliferation and migration.
A) Comparison of the proliferation of U87 cells in response to various doses of thrombospondin-1 as indicated. B) Representative images from three separate experiments of transwell migration assays in the presence or absence of the thrombospondin-1 (TSP; 1 nM), an activator of the α2δ-1 subunit, after 48 h of incubation. C) Comparative analysis of three transwell independent experiments as in B. Data show the mean ± SEM of three separate experiments. The asterisk denotes statistically significant differences with respect to the control condition (*P < 0.05 compared to untreated cells).
https://doi.org/10.1371/journal.pone.0279186.g005
Sp1 and TLR-4 overexpression increases cell proliferation and migration
Previous studies in human tumors have shown that Sp1 and TLR-4 participate in glioblastoma development; therefore, the overexpression of these proteins was subsequently evaluated separately in U87 glioblastoma cells to assess their role in cell proliferation and migration. The cell counting results showed that Sp1 and TLR-4 overexpression significantly increased cell proliferation, although to a lesser extent than observed after α2δ-1 overexpression (Fig 6A). Similarly, the overexpression of Sp1 and TLR-4 promoted cell migration, as observed in transwell chamber assays (Fig 6B). In this case, the effect is more evident than that produced by the overexpression of α2δ-1 (Fig 6C). These findings corroborate that α2δ-1 is directly involved in U87 cell proliferation and migration and suggest that the Sp1 and TLR-4 also play decisive roles in the proliferation and migration of glioblastoma cells.
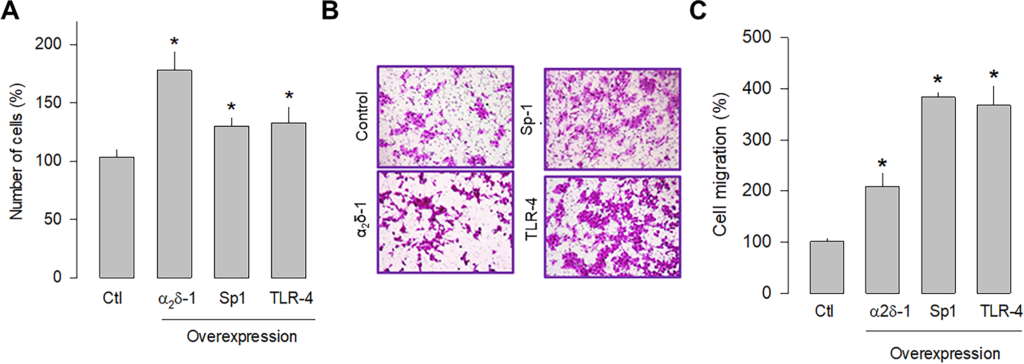
Effect of α2δ-1, Sp1, or TLR-4 overexpression on the proliferation and migration of U87 cells.
A) Comparison of cell number after transient transfection of U87 cells with α2δ-1 subunit, Sp1, and TRL-4 as assessed by direct cell counting. Values are expressed as the percentage of untransfected cells (Ctl), and each bar represents the mean ± SEM of triplicate determinations in 3 separate experiments. *P < 0.05 versus untransfected cells. B) Transwell migration assays of control and cells transiently transfected with α2δ-1, Sp1, or TLR-4 cDNA clones. Representative images from three independent experiments are shown. C) Comparative analysis of the mean ± SEM of 3 transwell separate experiments as in B. *P < 0.01 compared to untransfected cells.
https://doi.org/10.1371/journal.pone.0279186.g006
In order to independently verify whether α2δ-1, Sp1, and TLR-4 are related to cell proliferation and migration, a knockdown strategy for each of these proteins was then implemented. Hence, small interfering RNAs (siRNAs), were transfected into U87 cells before being subjected to proliferation (cell count) and migration (transwell chambers) assays. The results of this series of experiments showed that silencing α2δ-1, Sp1, or TLR-4 protein expression using specific siRNAs significantly decreased the number of cells (Fig 6A) and their migration capacity (Fig 7B and 7C). These data corroborate that α2δ-1, Sp1, and TLR-4 are related to glioblastoma cells’ proliferative and migratory capacity.
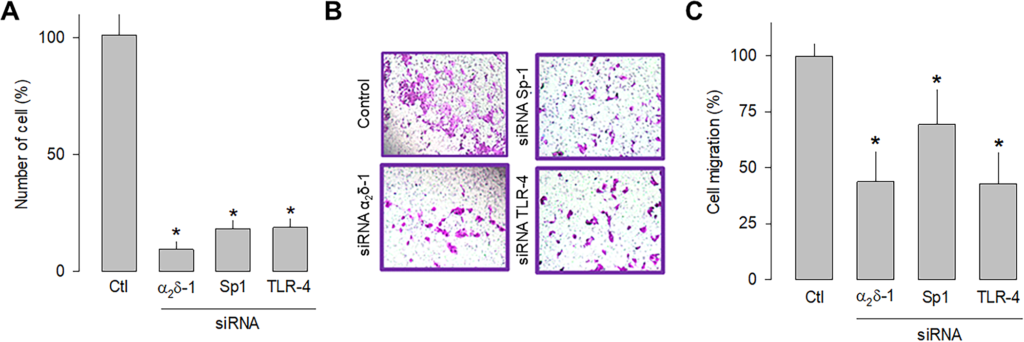
Effect of α2δ-1, Sp1, or TLR-4 silencing on the proliferation and migration of U87 cells.
A) Comparison of cell number after α2δ-1, Sp1, and TRL-4 knockdown with specific siRNAs of U87 cells as assessed by direct cell counting. Values are expressed as the percentage of untransfected cells (Ctl), and each bar represents the mean ± SEM of triplicate determinations in 3 separate experiments. *P < 0.01 versus untransfected cells. B) Transwell migration assays of control and cells transiently transfected with specific α2δ-1, Sp1, or TLR-4 siRNAs. Representative images from three separate experiments are shown. C) Comparative analysis of the mean ± SEM of 3 transwell independent experiments as in B. *P < 0.05 compared to untransfected cells.
https://doi.org/10.1371/journal.pone.0279186.g007
TLR-4 activation regulates α2δ-1 expression through the NF-kB/Sp1 signaling pathway
Last, we sought to determine which signaling pathway triggered by the activation of TLR-4 could be increasing the expression of Sp1 that finally resulted in the increased expression of α2δ-1. To this end, we first performed knockdown and overexpression experiments of TLR-4 and Sp1 separately using specific siRNAs and employing a siRNA scramble as a negative control and a siRNA against α2δ-1 as a positive control. Then, the effect of the experimental maneuver was evaluated by estimating protein expression by Western blot. This analysis showed that the silencing of both TLR-4 and Sp1 significantly decreased the expression of α2δ-1 (Fig 8A). Conversely, by inducing the overexpression of both TLR-4 and Sp1, the expression of α2δ-1 was significantly increased (Fig 8B).
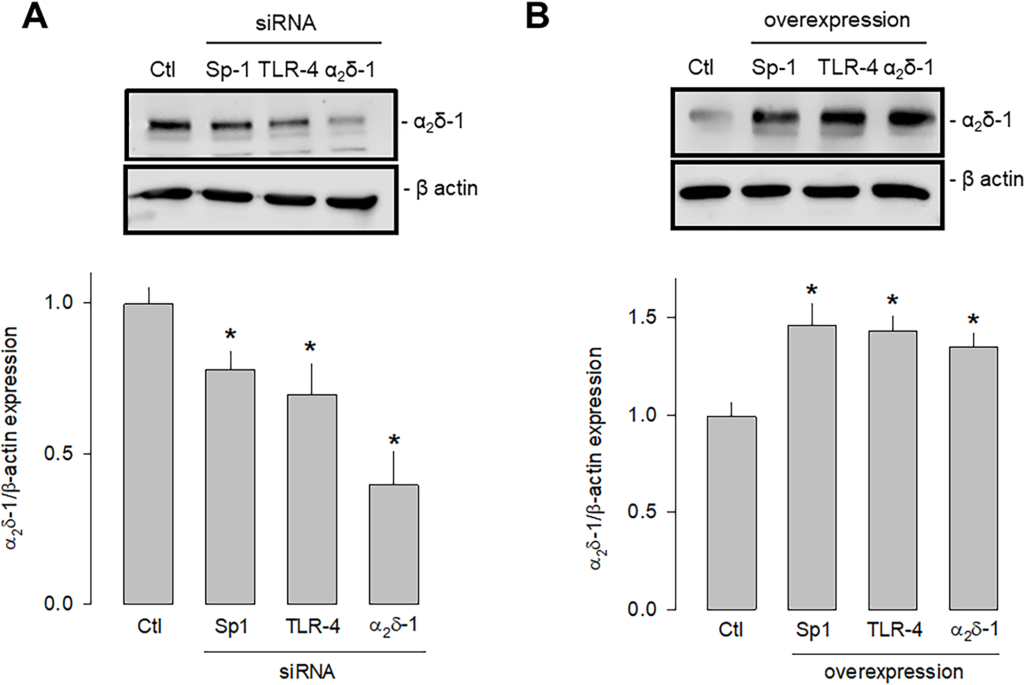
Effect of the knockdown and overexpression of TLR-4 and Sp1 on α2δ-1 expression.
A) Western blot assays performed on U87 cell lysates in the control condition and after transfection with the Sp1, TLR-4, and α2δ-1 specific siRNAs. The image shows a representative assay of three performed separately (upper panel). The signal obtained with the β-actin antibody served as the loading control. The lower panel shows the comparative densitometric analysis of the level of α2δ-1 protein. Data are shown as mean + SEM of three independent experiments in triplicate. B) Western blot assays performed on U87 cells in the control condition and after transfection with the Sp1, TLR-4, and α2δ-1 cDNA clones. The image shows a representative assay of three performed separately (upper panel). The signal obtained with the β-actin antibody served as the loading control. The lower panel shows the comparative densitometric analysis of the level of α2δ-1 protein. Data are shown as mean + SEM of 3 independent experiments in triplicate. *P < 0.05 compared to untransfected cells.
https://doi.org/10.1371/journal.pone.0279186.g008
The second series of experiments were carried out using an inhibitor of the nuclear factor кB (NF-кB) since it is known that the activation of TLR-4 involves two signaling pathways, a canonical one dependent on MyD88 (myeloid differentiation factor 88), and another non-canonical dependent on a protein called TRIF (Toll/IL-1R domain-containing adapter-inducing IFN-β). However, both pathways converge the nuclear translocation of NF-kB, affecting different transcription factors, including Sp1. For this reason, the expression of α2δ-1 and Sp1 was then evaluated in the presence and absence of the NF-κB inhibitor PDTC (pyrrolidine dithiocarbamate; Fig 9). These experiments showed that the inhibition of NF-kB significantly decreased the expression of both proteins. Moreover, this effect was maintained even in the presence of LPS, which supports the idea that the activation of TLR-4, which triggers the formation of the IKK/NF-κB complex, regulates the expression of Sp1 and in consequence the expression of α2δ-1.
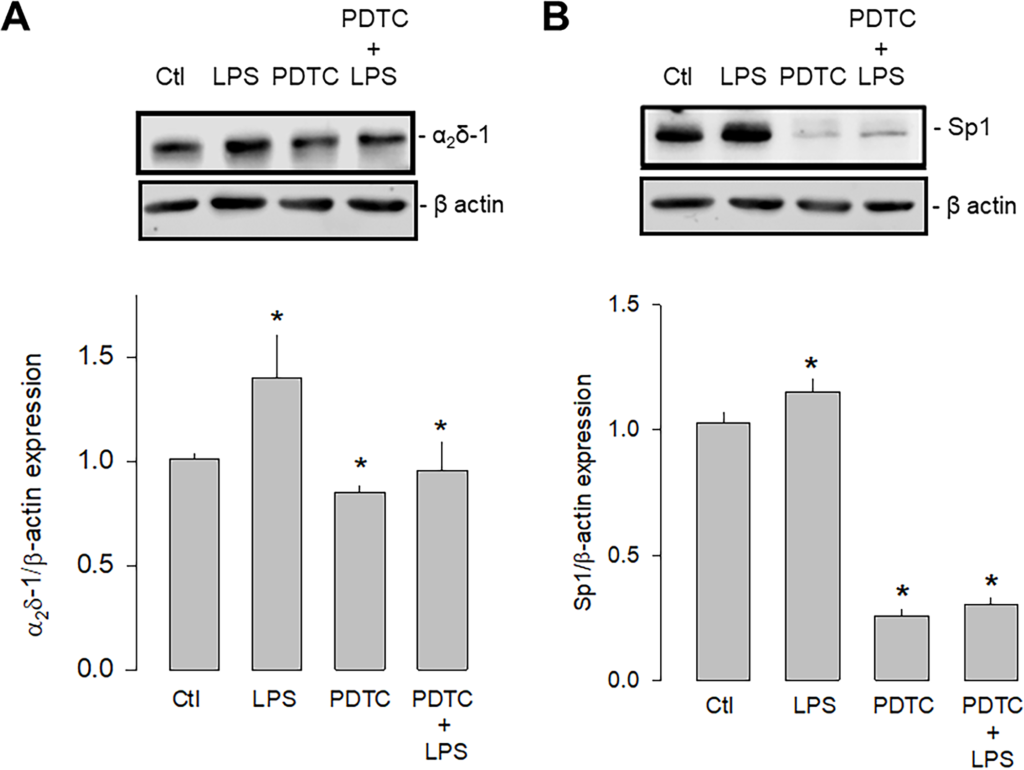
Effect of NF-кB inhibition on Sp1 and α2δ-1 expression.
A) Western blot assays performed on U87 cell lysates in the control condition and after treatment with the TLR-4 agonist LPS and the transcription factor NF-кB inhibitor PDTC. The image shows a representative assay of three performed separately (upper panel). The signal obtained with the β-actin antibody served as the loading control. The lower panel shows the comparative densitometric analysis of the level of α2δ-1 protein. Data are shown as mean + SEM of 3 independent experiments in triplicate. B) Sp1 Western blot assays performed on U87 cells in the conditions as in A. The image shows a representative assay of three performed separately (upper panel). The signal obtained with the β-actin antibody served as the loading control. The lower panel shows the comparative densitometric analysis of the level of Sp1 protein. Data are shown as mean ± SEM of 3 independent experiments in triplicate. *P < 0.05 compared to untreated cells.
https://doi.org/10.1371/journal.pone.0279186.g009
Discussion
The α2δ-1 subunit is a protein with complex topological features, having a molecular weight of ~170 kDa subject to post-translational proteolytic cleavage. Hence, the mature protein is composed of two peptides, α2 and δ, which in native conditions are kept together by a disulfide bond [34]. Although the central physiological role of α2δ-1 is to function as an auxiliary subunit of voltage-gated Ca2+ channels [30], its significant role in tumor progression has already been demonstrated previously in several types of cancer cell lines, including larynx, lung, ovary, liver, stomach, and breast [13–20]. However, its contribution to the onset and/or development of nervous system tumors remains virtually unexplored. In this report, we show evidence that TLR-4 activation by LPS increases the expression of the α2δ-1 subunit and promotes the proliferative and migratory potential of U87 human glioblastoma cells. Furthermore, our data suggest that TLR-4-mediated regulation of α2δ-1 expression occurs through the NF-kB/Sp1 signaling pathway.
Toll-like receptors (TLRs) signaling pathways play essential roles in the immune system controlling tumor growth and progression. TLRs activation may result in the production of cytokines, chemokines, interferons, and the transcription factor NF-κB. TLR-4 activation may be mediated by LPS, a bacterial endotoxin, leading to two distinct signaling pathways: the primary response pathway through MyD88 and the IFN-β (TRIF) pathway [35, 36]. Activation of TLR-4 may increase the production of factors that promote tumor development via the MyD88 pathway. MyD88 activates the IRAK kinase and interacts with the TRAF6 factor, resulting in the nuclear translocation of NF-κB, which, once in the nucleus, can interact and coactivate the Sp1 transcription factor promoting the transcription of genes, including that of the α2δ-1subunit.
It is worth mentioning here that voltage-gated ion channels in general, and Ca2+ channels in particular, play fundamental roles in controlling electrical signaling through the generation of nerve impulses [37]. In addition, however, it has been shown that these proteins can also contribute significantly to cellular biochemical signaling, helping to determine several physiological events, including cell division, cell cycle progression, and volume regulation [38]. All these functions are of great relevance for the proliferation of cancer cells [37–39].
The intracellular Ca2+ concentration is precisely and strictly controlled to generate Ca2+ signals with particular spatio-temporal attributes. Furthermore, this control is essential for the differential regulation of different proteins and Ca2+-dependent signaling pathways involved in specific cellular processes, including cell proliferation and migration [7, 39]. Since these functions are relevant for tumorigenesis, alterations in intracellular Ca2+ homeostasis and Ca2+signaling have been proposed to be crucial in driving or maintaining the malignant phenotypes. Indeed, tumor transformation is associated with changes in the expression and/or function of the molecules responsible for Ca2+ transport, which eventually controls many intracellular signaling pathways. This may result in the evasion of apoptosis with increased survival, excessive proliferation, malignant angiogenesis, cell migration, and metastasis [7]. In this context, the overexpression of α2δ-1 could be causing changes in membrane localization of CaV channels in U87 cells, which could give rise to changes in the Ca2+ concentration of different compartments and cellular microdomains and changes in intracellular signaling patterns.
On the other hand, it has been proposed that other α2δ subunits (of which there are four isoforms) are also associated with cancer development. For example, recent studies in prostate cancer have shown that α2δ-2 overexpression induces increased cell proliferation in vitro and that the overexpression of α2δ-2 LNCaP cell xenografts in nude mice is tumorigenic. In support of a direct role for α2δ-2 in prostate cancer development, gabapentin, an inhibitor of α2δ subunits, was capable of inhibiting tumor development in xenografts [40].** In contrast, the CACNA2D3 gene, which encodes for α2δ-3, has been identified as a methylation site in gastric cancer. Furthermore, CACNA2D3 methylation is associated with shorter survival, making it a useful prognostic marker for this type of cancer [41]. The potential tumor-inhibiting properties of α2δ-3 have also been observed in vitro, where overexpression results in reduced cell growth and adhesion, while its silencing using siRNAs had the opposite effect [41]. Likewise, the methylation-dependent silencing of CACNA2D3 has been proposed as a biomarker for the risk of metastasis in breast cancer [42]. These findings imply that, beyond the canonical role of the α2δ auxiliary subunits in determining the properties and functional diversity of the high-threshold voltage-gated Ca2+ channels, there may be some non-canonical molecular interactions not related to its primary function as part of the macromolecular complex of Ca2+channels. These interactions could be responsible, at least in part, for some of the actions of the α2δ-1 subunit on cell proliferation and migration.
Though the precise molecular mechanism by which the α2δ-1 subunit affects proliferation and migration in human glioblastoma cells, its cellular actions may be grouped into two general mechanisms. The first of them would be directly associated with the function of the subunit as part of the high-threshold Ca2+ channel complex, while the second would be independent of its role in the channels. The elucidation of which of these mechanisms, which are not mutually exclusive, contributes to a greater extent to the actions of α2δ-1 on cell proliferation and migration in glioblastoma cells, as well as the identification of molecular interactors for the α2δ-1 subunit outside the channel related to the control of cell proliferation and migration, are undoubtedly exciting topics for future research. In any case, the link found between the Ca2+channel auxiliary subunit and glioblastoma reveals this protein as a possible biomarker and/or therapeutic target. However, further mechanistic understanding of how the α2δ-1 contributes to tumor progression will be required before its diagnostic or therapeutic potential can be exploited.