Incorporation of a selective sigma-2 receptor ligand enhances uptake of liposomes by multiple cancer cells
By Yifei Zhang, Yixian Huang, Peng Zhang, Xiang Gao, Robert B Gibbs, and Song Li
Excerpt from the article published in International Journal of Nanomedicine, 7: 4473-4485, 07 Dec 2022, DOI: 10.2147/IJN.S31981
Editor’s Highlights
- Sigma 2-selective ligands can be employed for the targeted delivery of liposomal vectors.
- The tumor-targeting efficiency of Sigma 2-selective ligands has been demonstrated by conjugating SV119 with apoptosis-inducing peptides, which resulted in the selective killing of tumor cells.
Abstract
Background: The sigma-2 receptor is an attractive target for tumor imaging and targeted therapy because it is overexpressed in multiple types of solid tumors, including prostate cancer, breast cancer, and lung cancer. SV119 is a synthetic small molecule that binds to sigma-2 receptors with high affinity and specificity. This study investigates the utility of SV119 in mediating the selective targeting of liposomal vectors in various types of cancer cells.
Methods: SV119 was covalently linked with polyethylene glycol-dioleyl amido aspartic acid conjugate (PEG-DOA) to generate a novel functional lipid, SV119-PEG-DOA. This lipid was utilized for the preparation of targeted liposomes to enhance their uptake by cancer cells. Liposomes with various SV119 densities (0, 1, 3, and 5 mole%) were prepared and their cellular uptake was investigated in several tumor cell lines. In addition, doxorubicin (DOX) was loaded into the targeted and unmodified liposomes, and the cytotoxic effect on the DU-145 cells was evaluated by MTT assay.
Results: Liposomes with or without SV119-PEG-DOA both have a mean diameter of approximately 90 nm and a neutral charge. The incorporation of SV119-PEG-DOA significantly increased the cellular uptake of liposomes by the DU-145, PC-3, A549, 201T, and MCF-7 tumor cells, which was shown by fluorescence microscopy and the quantitative measurement of fluorescence intensity. In contrast, the incorporation of SV119 did not increase the uptake of liposomes by the normal BEAS-2B cells. In a time course study, the uptake of SV119 liposomes by DU-145 cells was also significantly higher at each time point compared to the unmodified liposomes. Furthermore, the DOX-loaded SV119 liposomes showed significantly higher cytotoxicity to DU-145 cells compared to the DOX-loaded unmodified liposomes.
Conclusion: SV119 liposomes were developed for targeted drug delivery to cancer cells. The targeting efficiency and specificity of SV119 liposomes to cancer cells was demonstrated in vitro. The results of this study suggest that SV119-modified liposomes might be a promising drug carrier for tumor-targeted delivery.
Introduction
One major problem with conventional anticancer agents is their severe side effects on normal tissues and organs. To solve this issue, targeted drug delivery to solid tumors was developed as an effective strategy to improve the therapeutic effect and minimize toxicity. Many factors affect the success rate of targeted drug delivery, including the choice of targeting ligands. Many types of ligands have been explored, including antibodies, peptides, aptamers, and small molecule ligands.1 Small molecule ligands have the advantages of minimal immunogenicity, excellent chemical stability, and ease of synthesis.2 Some examples of small molecule ligands that have been developed for tumor targeting include ligands of the folate receptor,3 prostate-specific membrane antigen (PSMA),4 and integrin receptors.5 There has been a recent interest in exploring sigma receptor ligands for tumor imaging and targeted therapy. Sigma receptors are overexpressed in a variety of human tumors including non-small cell lung carcinoma (NSCLC), prostate cancer, melanoma, and breast cancer.6–8 The small molecule ligands of sigma receptors are considered potential tumor imaging agents.6–8 The authors of this study and Dr Huang’s group have reported that sigma receptor ligands, such as anisamide, effectively mediate the selective delivery of liposomes containing doxorubicin (DOX) to prostate cancer cells in vitro and in vivo.9,10Extensive studies by Dr Huang’s group have demonstrated the targeted delivery of oligodeoxynucleotides and siRNA to lung cancer and melanoma using anisamide-decorated nanoparticles.11–13 Another sigma receptor ligand, haloperidol, was shown to improve the transfection of MCF-7 breast carcinoma cells by more than 10-fold, compared with the control (unmodified) liposomes.14
Neither anisamide nor haloperidol distinguishes between the sigma-1 or sigma-2 receptor subtypes with respect to binding. This study determines whether sigma 2-selective ligands can be employed for the targeted delivery of liposomal vectors. There are several potential advantages associated with sigma 2-selective ligands for tumor-targeted delivery. First, there is a higher density of sigma-2 versus sigma-1 receptors in tumor samples and cultured tumor cells.16–17 Second, ligand binding and photoaffinity labeling studies have demonstrated a lower expression of sigma-2 versus sigma-1 receptors in normal tissues from the central nervous system, gastrointestinal tract, kidney, liver, and heart.18 Third, a correlation between sigma-2 receptor expression and tumor proliferation has been established both in vitro and in vivo,19,20 but no such correlation was observed for the sigma-1 subtype.
SV119 is a small molecule ligand that binds to sigma-2 receptors with high affinity and specificity.21 The tumor-targeting efficiency of SV119 has been demonstrated by conjugating SV119 with apoptosis-inducing peptides, which resulted in the selective killing of tumor cells.22 The present study explores the potential of SV119 to mediate the targeted delivery of liposomes to multiple tumor cell lines. Liposomes are well-established drug carriers for intravenous delivery and several liposomal formulations have been approved for clinical applications.23 Our results show that the incorporation of a SV119 derivatized PEG-lipid significantly increases the cellular uptake of liposomes by several tumor cell lines, but not by normal cells. In addition, DOX-loaded SV119-modified liposomes showed significantly higher cytotoxicity compared to unmodified liposomes loaded with DOX. These findings suggest that SV119 liposomes may have potential for the targeted delivery of anticancer agents to tumors that over-express sigma-2 receptors.
Results and discussion
Several selective sigma-2 receptor ligands have recently been developed and evaluated in vitro and in vivo. For example, Mach and Wheeler reported the synthesis of a series of N-substituted 9-azabicyclo[3.3.1]nonan-3a-yl phenylcarbamate analogs.26 Several high-affinity sigma-2-selective ligands such as SV119 were identified through structure-activity relationship (SAR) studies. SV119 has a free amino group that is linked to a bridgehead nitrogen atom via a linker group of six methylene units. SAR studies suggest that the amino group is a preferred substituent for assuring a high affinity for σ2 receptors and high σ1 : σ2selectivity. Nevertheless, various SV119 conjugates were synthesized by chemically modifying this amino group. For example, coupling SV119 with a fluorescence molecule via this amino group led to the development of a sigma-2-selective probe – SW-120 – which was used to effectively assess the sigma-2 receptor expression levels in cultured tumor cells.27 In addition, a recent study by Spitzer et al showed that coupling SV119 with a proapoptotic peptide via this amino group similarly led to the development of a conjugate that demonstrated selective cytotoxicity towards tumor cells that overexpress the sigma-2 receptor.28
In order to develop lipid-based nanoparticles that incorporate SV119 on the surface, we designed and synthesized a SV119-derivatized PEG-lipid. DOA is a double-chain lipid that is synthesized from oleyl amine and aspartic acid and can be inserted into the lipid bilayer of liposomes. The PEG moiety on liposomes provides steric hindrance, which renders the nanoparticles more stable and minimizes nonspecific binding of the liposomes on the cell surface.29 After replacing the amine group with a carboxyl group, a SV119-PEG-DOA conjugate was synthesized by covalent linkage between the SV119 carboxyl group and the PEG terminal amine group. Figure 1 illustrates the synthesis scheme of SV119-PEG3500-DOA.
Figure 2A shows the NMR spectrum of synthesized SV119-PEG3500-DOA. The peaks at 7.2–7.3 ppm are attributed to the benzene groups on SV119, the signals at 5.0–5.2 ppm are attributed to the double bond of DOA, the peaks between 3–4 are attributed to the PEG, and the signals at 1–2 ppm are attributed to the carbon chain of DOA. The molecular weight of the synthesized product was determined by MALDI-TOF mass spectrometry. As shown in Figure 2B, the spectra exhibited a bell-shaped distribution of 44 Da-spaced lines centered at 4754.9 Da for SV119-PEG3500-DOA. A comparison between the mass spectrum of SV119-PEG3500-DOA (Figure 2B) with PEG3500 (Figure S2) and PEG3500-DOA (Figure S3) suggests the successful synthesis of the SV119-PEG3500-DOA conjugate.
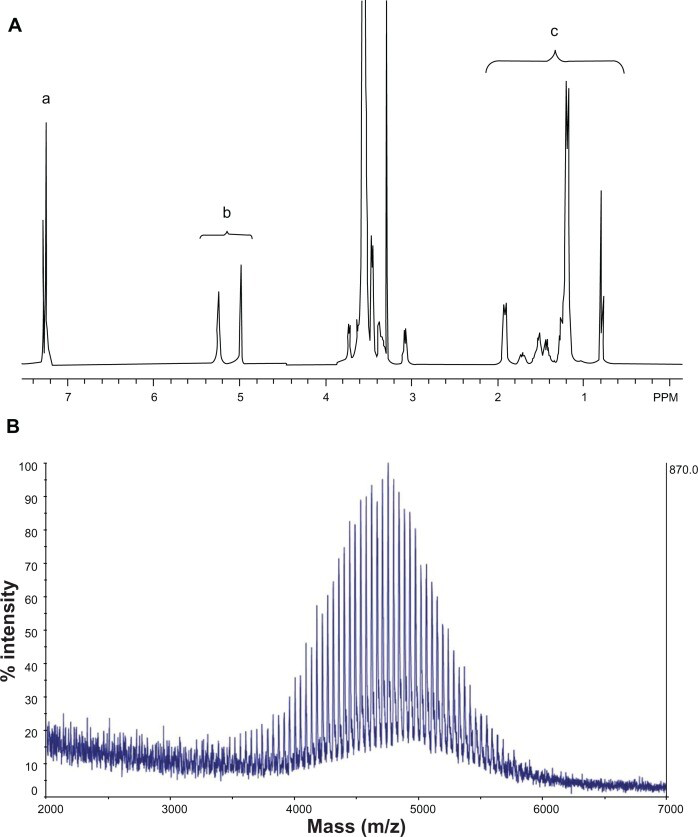
NMR spectrum (A) and MALDI-TOF mass spectrum (B) of SV119-PEG3500-DOA. The peaks in (A) are attributed to the benzene groups on SV119 (a), the double bonds of DOA (b), and the carbon chains of DOA (c).
Abbreviations: DOA, dioleyl amido aspartic acid; MALDI-TOF, matrix-assisted laser desorption/ionisation-time of flight; NMR, nuclear magnetic resonance; PEG, polyethylene glycol.
The synthesized SV119-PEG-DOA was used to formulate targeted liposomes. Liposomes are mainly composed of phospholipids and cholesterol. These molecules are also components of the cells and, thus, are believed to be biocompatible and non-toxic. The present experiment demonstrates that all the liposomal formulations have a mean diameter of approximately 90 nm (Table 1), which is optimal for penetrating the blood vessels surrounding the tumor and subsequent endocytosis by the tumor cells.30
SV119-PEG-DOA | 0% | 1% | 3% | 5% |
|---|---|---|---|---|
| Particle size (nm) | 90.0 ± 0.2 | 90.7 ± 0.3 | 97.6 ± 0.2 | 89.2 ± 0.6 |
| Zeta potential (mV) | − 2.0 ± 0.6 | − 2.4 ± 0.5 | − 2.2 ± 0.3 | − 3.1 ± 0.4 |
Size and zeta potential of SV119 liposomes
Note: Data are the mean ± SD for n = 3.
The targeting efficiency of SV119 liposomes was characterized in vitro. Cellular uptake was studied using DU-145 cells as target cells. DU-145 is a human prostate cancer cell line and these cells are reported to overexpress sigma-2 receptors.8 Human bronchial epithelial cells – BEAS-2B – were chosen to examine the liposomal uptake by normal cells. SV119-PEG3500-DOA was formulated into rhodamine-PE labeled liposomes to partially substitute PEG2000-DSPE, and the total amount of PEG-conjugated lipid in the liposomes was kept at 5 mole%. A longer spacer (PEG3500) was used between SV119 and DOA to minimize the potential steric hindrance imposed by DSPE-PEG2000 and to increase the targeting efficiency.31Figure 3A shows the fluorescence images of the DU-145 cells that were treated with rhodamine-PE labeled SV119 liposomes. After incubation with SV119 liposomes or unmodified liposomes for 1 hour, fluorescence was observed on the cell membrane and inside the cells. Increasing the amount of SV119 in the liposomes was associated with a significant increase in the intensity of rhodamine fluorescence. This is in contrast to the unmodified liposomes, which showed minimal binding and cellular uptake with the DU-145 cells (Figure 3A). Figure 3B shows the quantitative measurements of the cell-associated fluorescence 1 hour after the DU145 cells were treated with the SV119 liposomes and the unmodified liposomes. Consistent with the fluorescence imaging studies (Figure 3A), the amount of cell-associated fluorescence for the DU-145 cells increased significantly as the amount of ligand in the liposomes increased. In addition, the binding and uptake of SV119 liposomes was competitively blocked by the presence of the SV119-PEG550 conjugate, but was not inhibited by the same concentration of PEG550 (Figure S1B). These results demonstrate that the enhanced uptake of SV119 liposomes was mediated by SV119.
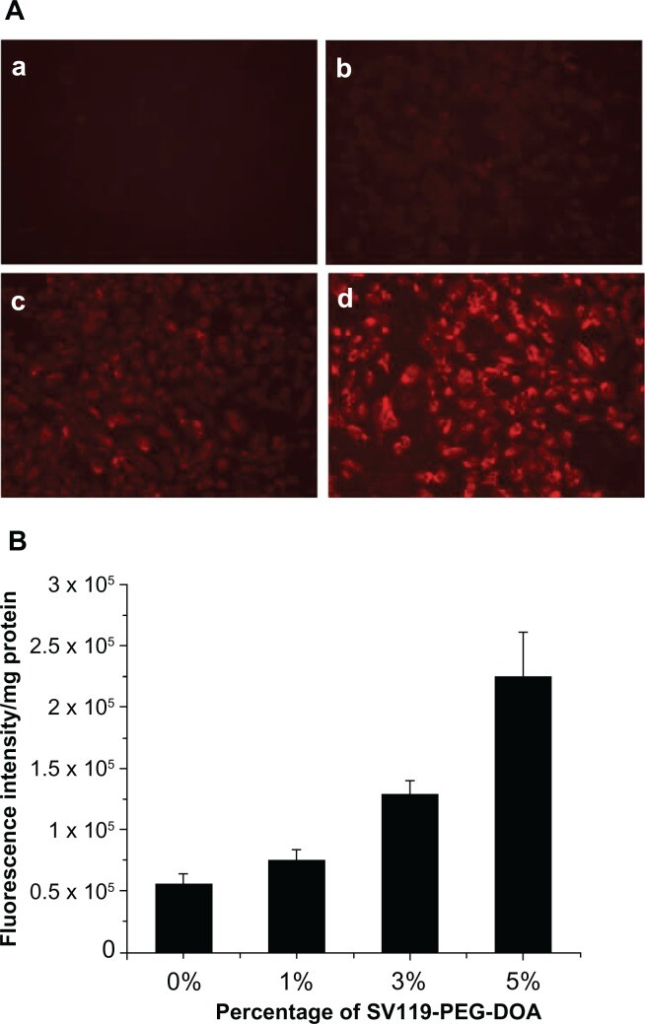
Enhanced intracellular uptake of SV119 liposomes by DU-145 cells, examined by microscopic study
(A) and quantitative analysis (B) (n = 4, mean ± SD). In (A), the percentage of SV119-PEG-DOA in the total lipid was 0% (a), 1% (b), 3% (c), and 5% (d), respectively.
Abbreviations: DOA, dioleyl amido aspartic acid; PEG, polyethylene glycol.
In order to demonstrate the cell-type specificity of the uptake of SV119 liposomes, normal human bronchial epithelial cells (BEAS-2B) were tested as a control. The results showed little binding and cellular uptake of SV119 liposomes and unmodified liposomes by the BEAS-2B cells (Figure 4A). No difference was found between the SV119 liposomes and unmodified liposomes in terms of the intensity of cell-associated fluorescence (Figure 4B). These observations suggest that SV119-mediated liposomal uptake is specific to tumor cells.
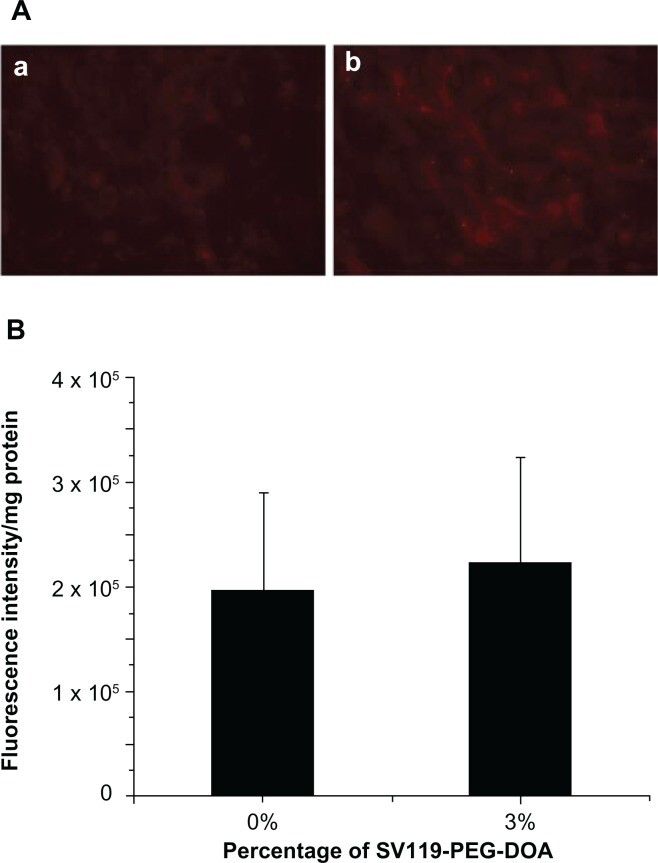
Cellular uptake of liposomes by BEAS-2B cells.
Microscopic images (A), and quantitative analysis (B) (n = 4, mean ± SD). The percentage of SV119-PEG-DOA in the total lipid was 0% (a), or 3% (b).
Abbreviations: DOA, dioleyl amido aspartic acid; PEG, polyethylene glycol.
One of the unique features of the sigma-2 receptor is its overexpression in multiple types of cancers. Therefore, following the demonstration of the specific targeting of DU145 cells, we examined the targeting capacity of SV119 liposomes using several other cancer cell lines, including PC-3 (prostate cancer), MCF-7 (breast cancer), A549, and 201T (lung cancer) cells. Similar to the DU-145 cells, the cell-associated fluorescence intensity of the SV119 liposomes was much higher than that of the unmodified liposomes in all the tumor cell lines tested (Figure 5), which suggests a potential for the SV119 liposomes to target multiple types of tumors.
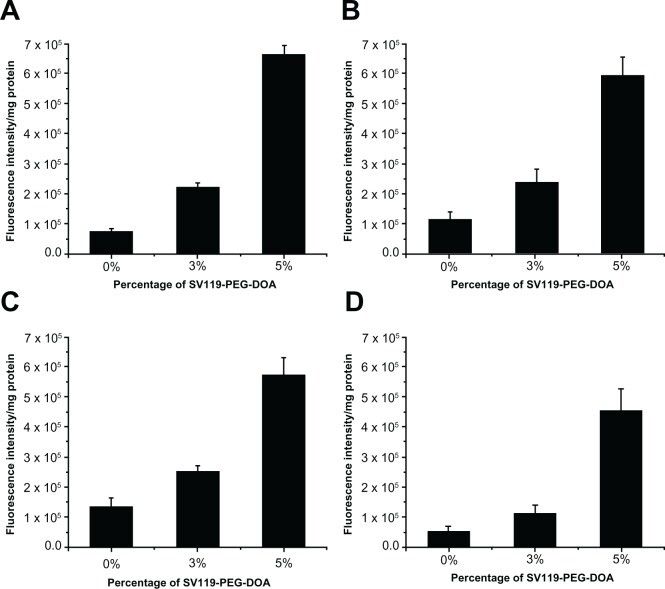
Uptake of SV119 liposomes by MCF-7
(A), PC-3 (B), 201T (C), and A549 cells (D) (n = 4, mean ± SD).
Abbreviations: DOA, dioleyl amido aspartic acid; PEG, polyethylene glycol.
Next, we investigated the time course of liposomal uptake by the DU-145 cells. As shown in Figure 6, the cell-associated fluorescence intensity increased during the prolonged incubation within 6 hours for the SV119 liposomes and the unmodified liposomes. Note that the cellular uptake of the SV119 liposomes was significantly higher at each time point compared to the unmodified liposomes. This observation further supports our conclusion that the incorporation of SV119 significantly enhances the uptake of liposomes into tumor cells.
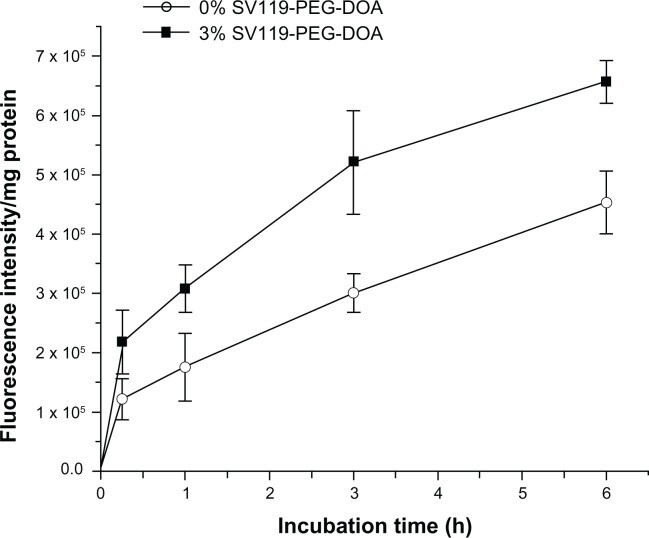
Time-course of cellular uptake for SV119 liposomes.
Notes: Rhodamine-PE labeled liposomes with 0% or 3% SV119-PEG-DOA were incubated with DU-145 cells for an indicated period at 37°C. (n = 4, mean ± SD).
Abbreviations: DOA, dioleyl amido aspartic acid; PEG, polyethylene glycol.
Like many other sigma-2 receptor ligands,26 SV119 has a tertiary amine group. Therefore, incorporating SV119 onto the surface of liposomes may positively charge the particles, which may increase the nonspecific cellular uptake of the SV119 liposomes. However, the zeta potential analysis showed that the incorporation of up to 5 mol% of lipid-PEG-derivatized SV119 into the liposomes did not significantly alter the surface charge (Table 1), which excluded the possibility that the enhanced uptake was mediated by a non-specific charge-charge interaction. Furthermore, to minimize the protonation of the amine nitrogen, a modified SV119 (mSV119) was synthesized by introducing an adjacent carbonyl group, and the mSV119 was similarly conjugated with PEG3500-DOA for incorporation into the liposomes (Figure 7A). This modification did not affect the targeting efficiency of the ligand (Figure 7B). This result may shed new light on the structure-activity relationship, which may aid in the design of new sigma-2 receptor ligands.
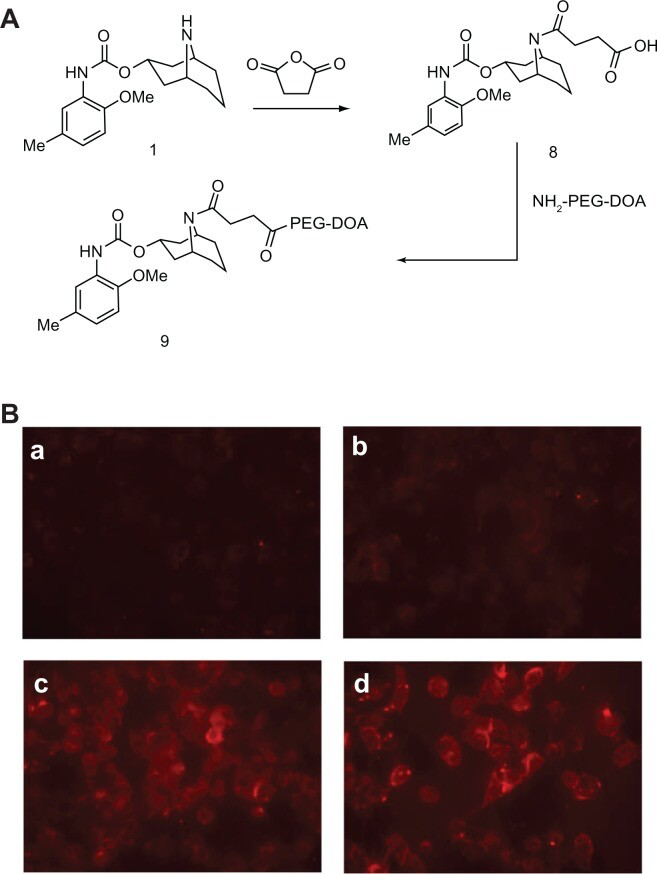
Uptake of liposomes decorated with modified SV119 (mSV119) by DU-145 cells. Scheme of synthesis
(A) and microscopic images (B) (n = 4, mean ± SD). In (B), the percentage of mSV119-PEG-DOA in the total lipid was 0% (a), 1% (b), 3% (c), and 5% (d), respectively.
Abbreviations: DOA, dioleyl amido aspartic acid; PEG, polyethylene glycol.
After demonstrating the efficiency and specificity of SV119 in mediating the uptake of liposomes by tumor cells, we investigated the utility of the SV119 liposomes for the targeted delivery of anticancer agents. We chose DOX because it is a chemotherapeutic drug widely used in clinical therapy. The DU-145 cells were pre-treated with DOX-loaded SV119 liposomes (SV119-L[D]), DOX-loaded unmodified liposomes (L[D]) and free DOX for 1 hour. The cells were then cultured for an additional 72 hours before the MTT assay was conducted. The results show that the cytotoxic effects of all the treatments were dose-dependent at concentrations of 0.2∼10 μg/mL (Figure 8). At each of the indicated concentrations, the viability of the cells treated with L[D] showed enhancements of 1.5–3-fold, compared with that of the free DOX, which suggests that the cells were protected by the encapsulation of the drug in the liposomes. In addition, SV119-L[D] exhibited an ∼2-fold higher cytotoxicity to the DU-145 cells than L[D] (Figure 8), which suggests improved efficacy of the SV119-modified liposomes versus the unmodified liposomes for delivering DOX to the tumor cells. The increased cytotoxicity of SV119-L[D] is consistent with the enhanced uptake of the SV119 liposomes, as shown in Figure 3. This demonstrates that the SV119 liposome can potentially be used a carrier for the targeted delivery of chemotherapeutics to tumor cells.
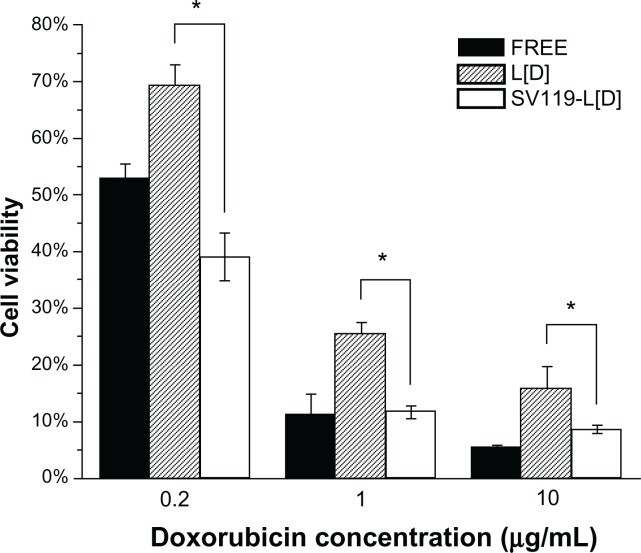
Cytotoxic effect of free and liposomal DOX on DU-145 prostate cancer cells.
Cells were treated with free DOX, DOX-loaded SV119 liposomes or DOX-loaded unmodified liposomes with DOX concentrations of 0.2, 1, or 10 μg/mL. Cytotoxicity was assessed by MTT assay. Cells receiving no treatment were defined as the maximal cell viability. Values presented are the mean ± SD of six replicates.
Note: *P < 0.05 compared to the control treatments with unmodified liposomes.
Abbreviations: DOX, doxorubicin; L[D], DOX-loaded unmodified liposomes.
Despite the enhanced cellular uptake of the SV119 liposomes, it is crucial to understand the intracellular fate of the liposomes in order to maximize the bioavailability of the loaded drug. Nanoparticles internalized via the endocytosis pathway are often sequestered in endosomes and lysosomes, which represent an intracellular barrier that limits the accessibility of encapsulated agents to reach their molecular targets. In this study, confocal microscopy was used to examine the intracellular localization of rhodamine-PE labeled SV119 liposomes in DU-145 cells. After 3 hours of incubation, the liposomes showed a peri-nuclei punctuated distribution that is substantially co-localized with early endosomes (Figure 9A–C). After 13 hours of additional incubation, the liposomes were largely accumulated in the lysosomes, as evidenced by the colocalization with the lysosome markers (LysoTracker®; Figure 9D–F). These observations are consistent with the internalization via the endocytosis pathway. Future studies are warranted to improve the formulation and to achieve more efficient release from the endosomes and lysosomes.
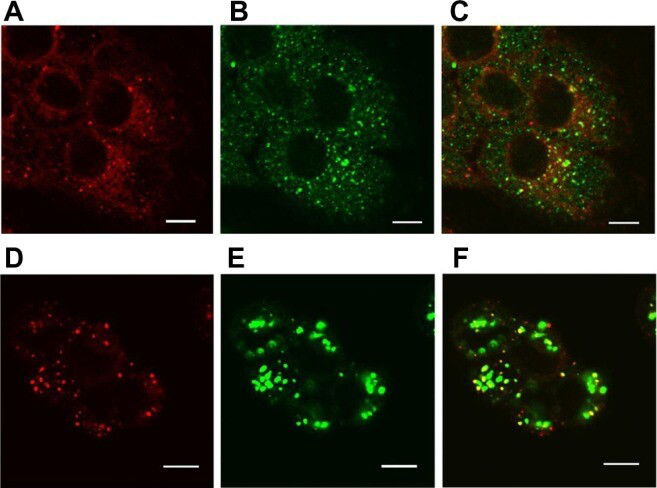
Localization of SV119 liposomes in DU-145 cells.
Cells were incubated with rhodamine-PE labeled SV119 liposomes for 3 hours (A–C) or 16 hours (D–F) in a serum-free medium, stained with markers of early endosomes (anti-EEA1; A–C) or lysosomes (LysoTracker®; Life Technologies, Carlsbad, CA) (D–F), and imaged using confocal microscopy. Red fluorescence represents rhodamine-PE labeled liposomes (A and D), green fluorescence represents early endosomes B) or lysosomes (E). Yellow color observed in the red + green overlay (C and F) indicates colocalization of red liposomes with the green endosomes (C) or lysosomes (F).
Note: Scale bar = 10 μm.
Conclusion
We have demonstrated the successful synthesis of the functional lipid, SV119-PEG-DOA, as well as its incorporation into a liposome formulation for targeted delivery to cancer cells. As compared with unmodified liposomes, the SV119 liposomes demonstrated enhanced uptake in multiple types of tumor cell lines including DU-145, MCF-7, PC-3, 201T, and A549 cells. In addition, this enhanced uptake was shown to be dependent on ligand density and incubation time, and the uptake of SV119 liposomes was dramatically inhibited by the presence of free SV119. In contrast, the enhanced uptake of SV119 liposomes was not observed in BEAS-2B normal cells, which suggests that enhanced uptake is tumor cell-specific. Furthermore, results of the MTT assay suggest that incorporating SV119 onto the surface of liposomes significantly enhances the delivery and cytotoxicity of liposomal DOX in vitro. At the time of writing this manuscript, Xia and colleagues reported that SV119 mediated the selective delivery of SV119-gold nanocage conjugates to tumor cells.32Collectively, these data suggest that SV119-conjugated nanoparticles may represent a promising delivery system for the targeted delivery of anticancer therapeutics to tumor cells.