Calcium current modulation by the γ1 subunit depends on alternative splicing of CaV1.1
By Yousra El Ghaleb, Nadine J. Ortner, Wilfried Posch, Monica L. Fernández-Quintero, Wietske E. Tuinte, Stefania Monteleone, Henning J. Draheim, Klaus R. Liedl, Doris Wilflingseder, Jörg Striessnig, Petronel Tuluc, Bernhard E. Flucher, and Marta Campiglio
Excerpt from the article published by Journal of General Physiology, 5 September 2022; 154 (9): e202213182. DOI: https://doi.org/10.1085/jgp.202113028
Editor’s Highlights
- CaV1.1 is a multiprotein complex consisting of a pore-forming α1 subunit and several auxiliary proteins: the intracellular β1a, the glycosylphosphatidylinositol-anchored extracellular α2δ-1, and the transmembrane γ1 subunits.
- α2δ-1 and γ1 are dispensable for functional expression of CaV1.1 in muscle cells but displayed an inhibitory effect on CaV1.1 currents.
- Whereas the α2δ-1 subunit slows down the activation, the γ1 subunit promotes voltage-dependent inactivation at more negative voltages.
- The γ1 subunit on the two splice variants of CaV1.1 in heterologous cells revealed multiple functions of γ1 in membrane targeting and functional modulation of the skeletal muscle calcium channel.
- Inclusion of exon 29 in CaV1.1a appears to allosterically render the channel susceptible to the reduction of its currents by γ1, as well as to the simultaneous relief of this block by RYR1.
Abstract
The skeletal muscle voltage-gated calcium channel (CaV1.1) primarily functions as a voltage sensor for excitation–contraction coupling. Conversely, its ion-conducting function is modulated by multiple mechanisms within the pore-forming α1S subunit and the auxiliary α2δ-1 and γ1 subunits. In particular, developmentally regulated alternative splicing of exon 29, which inserts 19 amino acids in the extracellular IVS3-S4 loop of CaV1.1a, greatly reduces the current density and shifts the voltage dependence of activation to positive potentials outside the physiological range. We generated new HEK293 cell lines stably expressing α2δ-1, β3, and STAC3. When the adult (CaV1.1a) and embryonic (CaV1.1e) splice variants were expressed in these cells, the difference in the voltage dependence of activation observed in muscle cells was reproduced, but not the reduced current density of CaV1.1a. Only when we further coexpressed the γ1 subunit was the current density of CaV1.1a, but not that of CaV1.1e, reduced by >50%. In addition, γ1 caused a shift of the voltage dependence of inactivation to negative voltages in both variants. Thus, the current-reducing effect of γ1, unlike its effect on inactivation, is specifically dependent on the inclusion of exon 29 in CaV1.1a. Molecular structure modeling revealed several direct ionic interactions between residues in the IVS3-S4 loop and the γ1subunit. However, substitution of these residues by alanine, individually or in combination, did not abolish the γ1-dependent reduction of current density, suggesting that structural rearrangements in CaV1.1a induced by inclusion of exon 29 may allosterically empower the γ1subunit to exert its inhibitory action on CaV1.1 calcium currents.
Introduction
Excitation–contraction (EC) coupling in skeletal muscle is initiated by action potentials that activate the voltage-gated calcium channel CaV1.1 located in the transverse tubules (T-tubules). In adult skeletal muscle, CaV1.1 functions as a voltage sensor that triggers the opening of the calcium release channel, the ryanodine receptor (RYR1), in the SR via protein–protein interactions, thus initiating muscle contraction (Rios and Brum, 1987; Schneider and Chandler, 1973). Because of the conformational coupling between CaV1.1 and RYR1, CaV1.1 currents are dispensable for skeletal muscle EC coupling (Armstrong et al., 1972; Dayal et al., 2017). Accordingly, in mammals, CaV1.1 channels activate only upon strong, non-physiological membrane depolarization and conduct small and slowly activating currents (Tanabe et al., 1988). This is strikingly different in the embryonic splice variant (CaV1.1e), which lacks 19 amino acids in the extracellular loop connecting segments S3 and S4 in the fourth homologous repeat, owing to alternative splicing excluding exon 29 (Tuluc et al., 2009). The embryonic CaV1.1e isoform activates upon physiological membrane depolarization and conducts currents that are substantially larger in amplitude than those of the adult CaV1.1a isoform.
CaV1.1 is a multiprotein complex consisting of a pore-forming α1 subunit and several auxiliary proteins: the intracellular β1a, the glycosylphosphatidylinositol-anchored extracellular α2δ-1, and the transmembrane γ1 subunits (Curtis and Catterall, 1984; Zamponi et al., 2015). While the β1a subunit was shown to be essential for the functional expression of CaV1.1 and for EC coupling (Gregg et al., 1996; Schredelseker et al., 2005), α2δ-1 and γ1 are dispensable for functional expression of CaV1.1 in muscle cells but displayed an inhibitory effect on CaV1.1 currents (Freise et al., 2000; Obermair et al., 2005; Held et al., 2002; Ursu et al., 2001; Arikkath et al., 2003; Tuluc et al., 2009; Ahern et al., 2001). The α2δ-1 subunit slows down the kinetics of activation of CaV1.1 currents, whereas the γ1 subunit reduces the current amplitude and shifts the voltage dependence of inactivation. However, neither the α2δ-1 nor the γ1 subunit is essential for EC coupling. In their absence, the amplitude and voltage dependence of the depolarization-induced calcium transients are unchanged (Obermair et al., 2005; Ahern et al., 2001; Ursu et al., 2004).
All the cited studies were performed in skeletal muscle cells using a knockout or knockdown approach since CaV1.1 expresses poorly in mammalian non-muscle cells. Whereas coexpression of the auxiliary subunits β and α2δ is sufficient to support functional expression of all other high voltage activated calcium channels (Singer et al., 1991; Lacerda et al., 1991; Zamponi et al., 2015), CaV1.1 coexpression with these subunits does not yield functional currents in heterologous cell systems. Only recently, it was demonstrated that the skeletal muscle–specific adaptor protein STAC3 is essential for membrane expression and robust currents of CaV1.1 in heterologous cells (Polster et al., 2015; Wu et al., 2018).
In the present study, we generated two HEK cell lines stably expressing the three subunits (STAC3, β3, and α2δ-1) necessary to support functional membrane expression of CaV1.1. These cell lines provide a unique tool for analysis of wild type and mutant CaV1.1 channel currents and pharmacology in non-muscle cells. Interestingly, in contrast to what had been reported in myotubes, our current analysis of the adult and embryonic CaV1.1 splice variants in the STAC3-HEK cell lines revealed no difference in their current densities, but still displayed the typical differences in voltage dependence of activation. Because coexpression of γ1 inhibits gating of CaV1.1a calcium currents in skeletal myotubes and tsA201 cells (Polster et al., 2016; Freise et al., 2000; Ahern et al., 2001), and because the recently resolved CaV1.1 structure revealed an interaction of γ1 subunit with the IVS3-S4 loop of CaV1.1a (Wu et al., 2016; Wu et al., 2015), we hypothesized that regulation of the gating properties of CaV1.1 channels by the γ1 subunit occurs in a splice variant–dependent manner. Indeed, we found that coexpressed γ1 subunits selectively reduced the current density of the adult CaV1.1a isoform, and not that of the embryonic CaV1.1e isoform. In contrast, γ1 similarly shifted the voltage dependence of steady-state inactivation to more negative voltages and increased CaV1.1 membrane expression of both isoforms. Molecular modeling predicted several ionic interactions between the γ1 subunit and the IVS3-S4 linker of CaV1.1a. However, site-directed mutagenesis of the putative ion-pair partners did not abolish γ1-dependent inhibition of the CaV1.1a currents, suggesting an allosteric effect of exon 29 that is important for modulation of current density by the γ1 subunit.
…
Results
Generation of two HEK cell lines expressing β3, α2δ-1, and STAC3
In order to generate HEK293 cell lines that could reliably support CaV1.1 expression, we inserted STAC3 into the genome of a host cell line already available, stably expressing α2δ-1 and β3, using the Flp-In T-Rex system. We generated two cell lines: one in which the expression of STAC3 was constitutive (HEK-STAC3) and one in which the expression of STAC3 was DOX inducible (HEK-TetOn-STAC3). While the parental HEK293 cell line showed neither STAC3 mRNA nor protein expression, the selected clone of the constitutive HEK-STAC3 cell line strongly expressed STAC3 (Fig. 1). As expected, without DOX induction, the selected clone of the inducible HEK-TetOn-STAC3 cell line showed only weak basal STAC3 mRNA and protein expression. However, 24 h after the beginning of DOX induction, STAC3 expression levels were strongly increased and comparable to those of the constitutive HEK-STAC3 cell line (Fig. 1).
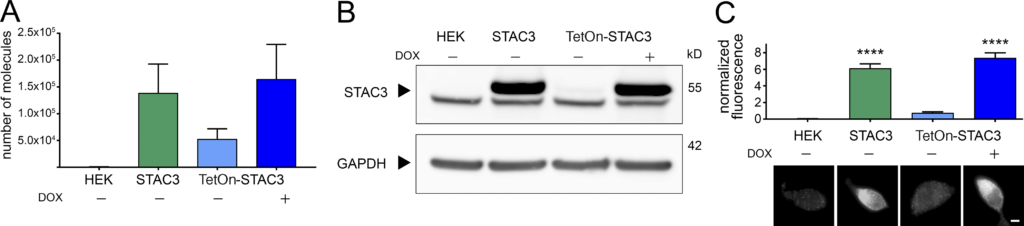
Both the constitutive and the inducible STAC3-HEK cell lines robustly express STAC3. (A)
STAC3 mRNA transcription levels in the host (HEK), the constitutive (STAC3), and the inducible (TetOn-STAC3) cell line, before and after DOX treatment, assessed by TaqMan quantitative PCR. Mean values of three replicates. (B) Western blot analysis with anti-STAC3 antibody indicated that STAC3 is substantially expressed by the constitutive and inducible cell lines (treated with DOX), while it is absent from the host cell line (HEK). Without DOX, the inducible cell line shows very low basal expression. An unspecific band present in all samples migrates slightly faster than STAC3. One representative experiment of three is shown. (C)Quantification of STAC3 staining intensity in the host (HEK), the constitutive (STAC3), and the inducible cell line (TetOn-STAC3), before and after DOX treatment reveals strong STAC3 expression in both STAC3 and TetOn-STAC3 cell lines. Scale bar, 2 µm. ANOVA, F(3,169) = 67.72; P < 0.0001; Tukey post hoc analysis; ****, P < 0.0001. Source data are available for this figure: SourceData F1.
We then analyzed the ability of the cell lines to support the expression of functional CaV1.1 currents by transient transfection with the adult CaV1.1a or the embryonic CaV1.1e isoforms. The two CaV1.1 isoforms differ in the length of the linker connecting helices S3 and S4 of the fourth homologous repeat, with the embryonic isoform skipping exon 29 and lacking 19 amino acids. Although both isoforms support skeletal muscle EC coupling, they display very different current properties when expressed in dysgenic (CaV1.1-null) myotubes. In contrast to the adult CaV1.1a isoform, the embryonic CaV1.1e splice variant activates at more hyperpolarizing potentials and conducts calcium currents that are several-fold larger than those of CaV1.1a (Tuluc et al., 2009). Our experiments show that both the constitutive (HEK-STAC3) and the inducible (HEK-TetOn-STAC3) cell lines efficiently supported functional expression of both the adult and the embryonic CaV1.1 variants (Fig. 2, A, B, E, and F; and Table 1). More interestingly, while the two CaV1.1 splice variants displayed the expected difference in the V1/2 of activation (Fig. 2, C, D, G, and H; and Table 1), the expected smaller current density in CaV1.1a was not observed in the two STAC3-HEK cell lines (Fig. 2, A, B, E, and F; and Table 1).
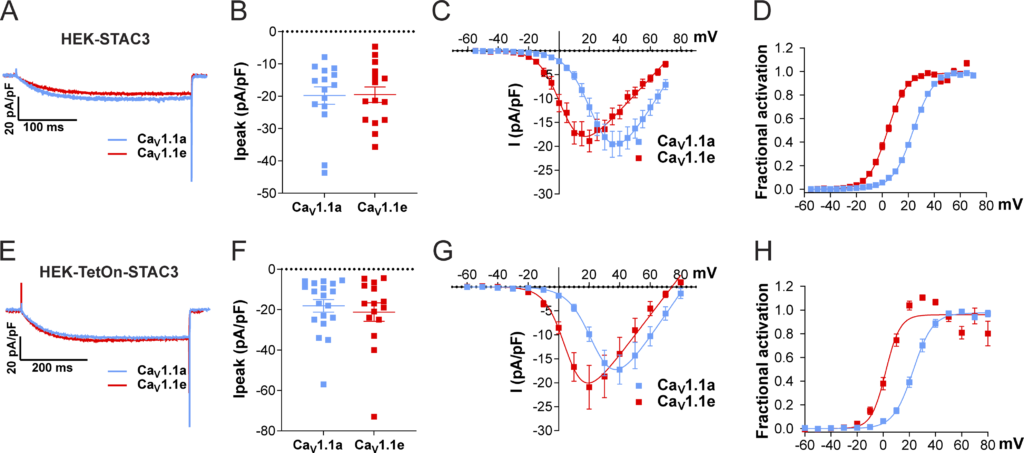
Exclusion of exon 29 in Ca V 1.1e shifts the voltage dependence of activation to more negative voltages but does not affect current density in either of the two STAC3-HEK cell lines. (A–D)
Current properties of CaV1.1a (blue, n = 15) compared with CaV1.1e (red, n = 15) in the HEK-STAC3 cell line. (E–H) Current properties of CaV1.1a (blue, n = 19) compared with CaV1.1e (red, n = 15) in the inducible cell line HEK-TetOn-STAC3 treated with DOX. (A and E) Exemplary current traces at Vmax show similar activation kinetics of the CaV1.1a and CaV1.1e variants and no difference in the peak current density (Ipeak; peak current normalized to the cell size) in both the HEK-STAC3 (B) and HEK-TetOn-STAC3 (F) cell lines (P = 0.94 and P = 0.56, respectively). The current–voltage relationship (C and G) and the normalized steady-state activation curves (D and H) show that exclusion of exon 29 (in CaV1.1e) results in a 20.4- and 21.1-mV left shift of activation when expressed in the HEK-STAC3 and HEK-TetOn-STAC3 cell line, respectively. Mean ± SEM; P values calculated with Student’s t test (see Table 1 for parameters and statistics).
We reasoned that some factor is missing in HEK cells that specifically mediates the splicing-dependent effect on the current amplitude in muscle cells. In muscle, the specific function of exon 29 is to curtail the calcium currents, and in our STAC3-HEK cells the currents were equally large, so the missing factor might be a muscle-specific protein capable of diminishing CaV1.1 currents specifically in the adult splice variant. The only CaV1.1 subunit not present in our expression system is the γ1subunit. Moreover, the γ1 subunit acts as a negative regulator of CaV1.1 currents both in skeletal muscle and in tsA201 cells (Freise et al., 2000; Ahern et al., 2001; Andronache et al., 2007; Polster et al., 2016), and its expression is restricted to skeletal muscle (Biel et al., 1991; Jay et al., 1990). Therefore, we inferred that the γ1subunit may be the missing factor selectively reducing the currents of CaV1.1a and not those of CaV1.1e. This notion was further supported by the fact that cryo-EM structures of CaV1.1 predicted an interaction of the γ1 subunit with the CaV1.1 IVS3-S4 region, exactly the site containing the alternatively spliced exon 29 (Wu et al., 2015, 2016).
The γ1 subunit selectively reduces the current density of CaV1.1a but not that of CaV1.1e
To test this hypothesis, we measured the calcium current density of CaV1.1a and CaV1.1e in the presence and the absence of γ1 in one of the newly established cell lines (HEK-TetOn-STAC3). As previously reported (Polster et al., 2016; Freise et al., 2000), the presence of γ1 significantly reduced CaV1.1a current amplitudes, with no significant effect on the voltage dependence of activation (Fig. 3, A–D; and Table 2). The activation kinetics were unaltered by coexpression of the γ1 subunit (Fig. S1, A–D; and Table 2), in agreement with what had been observed in myotubes (Freise et al., 2000) but contrary to what was previously reported in tsA201 cells (Polster et al., 2016). More importantly, in contrast to CaV1.1a, coexpression of γ1 had no effect on the current density of CaV1.1e (Fig. 3, E–H; and Table 2), suggesting that the inclusion of the 19 amino acids encoded in exon 29 is essential for suppression of the CaV1.1 current by the γ1 subunit.
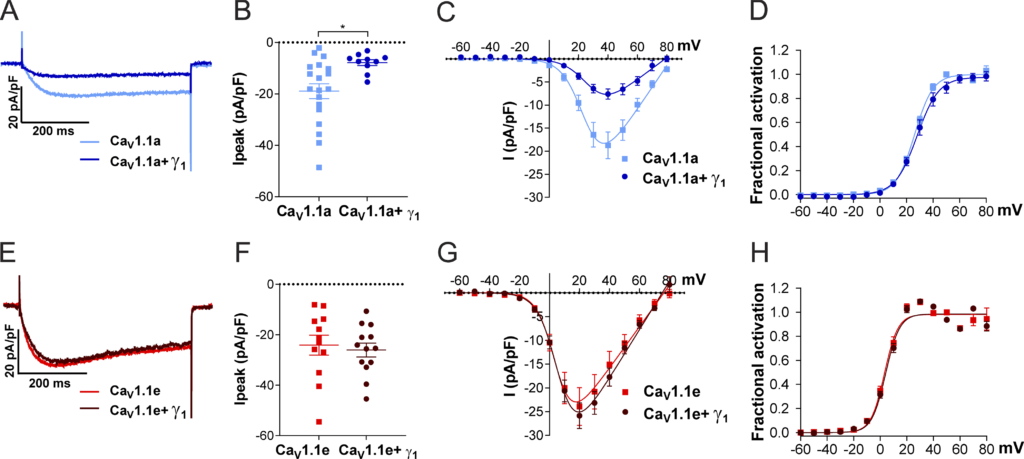
Coexpression of γ 1 reduces the current density in Ca V 1.1a but not in Ca V 1.1e. (A–D)
Current properties of the adult splice variant CaV1.1a (blue, n = 19) compared with CaV1.1a coexpressed with γ1 (CaV1.1a + γ1, dark blue, n = 10). (E–H) Current properties of the embryonic splice variant CaV1.1e (red, n = 12) compared with CaV1.1e + γ1 (dark red, n = 13). (A–H)Exemplary current traces at Vmax (A) and the scatter plot of the peak current density (Ipeak; B) show a significant reduction (P = 0.012) when coexpressing γ1 with CaV1.1a. In contrast, when coexpressing γ1 with CaV1.1e (E and F), no difference in current density was observed (P = 0.69). The current–voltage relationship (C and G) and the fractional steady-state activation curves (D and H) show no effect of γ1 on the voltage dependence of activation when coexpressed with CaV1.1a or CaV1.1e. Mean ± SEM; P values calculated with Student’s t test. *, P <0.05 (for parameters and statistics, see Table 2).
The γ1 subunit shifts the steady-state inactivation to more negative potentials in both CaV1.1 isoforms
The γ1 subunit inhibits CaV1.1 currents not only by decreasing the current amplitude, but also by promoting inactivation. In fact, previous studies demonstrated that, in the presence of γ1, the voltage dependence of inactivation shifted toward more negative potentials, while the voltage dependence of activation remained unaltered (Ahern et al., 2001; Freise et al., 2000; Held et al., 2002; Ursu et al., 2004).
To determine whether this γ1 effect on CaV1.1 currents is also restricted to the adult CaV1.1a isoform, we performed a steady-state inactivation protocol comparing the current size of test pulses before and after 45-s conditioning prepulses at incrementally increasing potentials (Fig. 4 A, inset). The normalized steady-state inactivation was plotted as a function of the prepulse potential. As previously demonstrated, coexpression of the γ1 subunit resulted in a robust left shift in the voltage dependence of inactivation of the adult CaV1.1a isoform (Fig. 4 A). In the presence of γ1, the half-maximal inactivation potential was shifted by 14.1 mV toward more hyperpolarizing potentials (Fig. 4 B and Table 2).
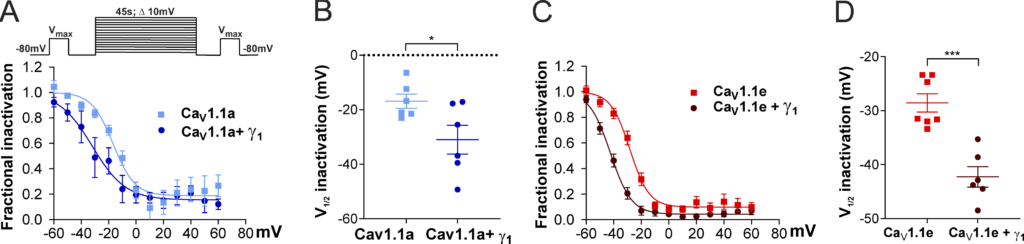
γ 1 L eft shifts the steady-state inactivation of both Ca V 1.1a and Ca V 1.1e currents. (A–D)
Steady-state inactivation of CaV1.1a (A and B) and CaV1.1e (C and D) currents in the presence or in the absence of γ1. The inset in A shows the steady-state inactivation protocol. Fractional inactivation curves and scatter plot of V1/2 of CaV1.1a currents (blue, n = 6) compared with CaV1.1a + γ1 currents (dark blue, n = 6; A and B); the same for CaV1.1e (red, n = 7) and CaV1.1e + γ1 currents (dark red, n = 6; C and D). The voltage dependence of inactivation is left shifted in CaV1.1a + γ1 (14.1 mV, P = 0.04) and CaV1.1e + γ1 (13.7 mV, P < 0.001). Mean ± SEM; P values calculated with Student’s t test. *, P < 0.05; ***, P < 0.001.
Surprisingly, these effects were recapitulated with the embryonic CaV1.1e isoform. In the presence of the γ1 subunit, the half-maximal inactivation potential was shifted to hyperpolarizing potentials by 13.7 mV (Fig. 4, C and D; and Table 2). These results suggest that, although the γ1 subunit fails to suppress the current of the embryonic CaV1.1e splice variant by reducing its amplitude (Fig. 3, E–G), it still inhibits CaV1.1e currents by left-shifting the steady-state inactivation.
The γ1 subunit was also reported to accelerate the inactivation kinetics of CaV1.1 (Ahern et al., 2001; Freise et al., 2000). Accordingly, the time constant of inactivation was reduced to 67% in the presence of γ1 for both CaV1.1a and CaV1.1e, although not to a statistically significant extent (Fig. S1, E–H; and Table 2).
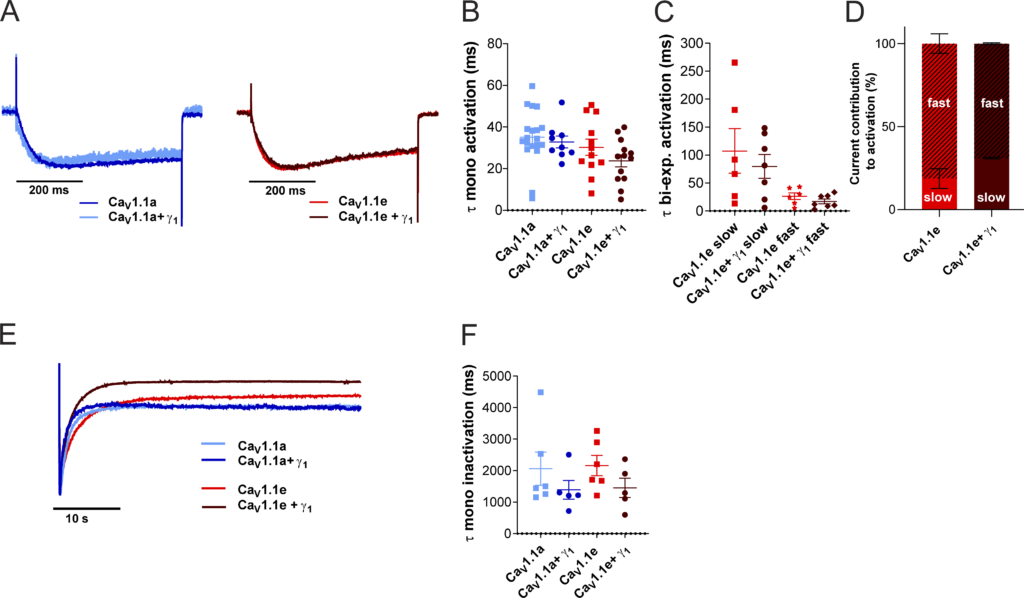
γ 1 Does not affect activation kinetics but shows a trend of accelerated inactivation kinetics. (A–D)
Time constants of activation of CaV1.1a (blue, n = 19), CaV1.1a + γ1 (dark blue, n = 9), CaV1.1e (red, n = 12), and CaV1.1e + γ1 (dark red, n = 13) of a monoexponential and biexponential fit (CaV1.1e) on the rising phase of the inward calcium current during a 500-ms depolarization to Vmax. (A) Example traces of 500-ms depolarization to Vmax in CaV1.1a (left) and CaV1.1e (right), normalized to the peak current. (B and C) No differences were found between the time constant of activation of CaV1.1a or CaV1.1e with or without γ1 coexpression when fitted monoexponentially (B) or between the fast or slow time constant of CaV1.1e (n = 6) and CaV1.1e + γ1 (n = 7) of the recordings that could be fitted biexponentially (C); CaV1.1a and CaV1.1a + γ1 could be fitted only monoexponentially. (D) The current contribution of the fast component was bigger than that of the slow component in both CaV1.1e (slow:fast ≈20:80) and CaV1.1e + γ1 (slow:fast ≈30:70), but the ratios were similar (P = 0.41). (E and F) Time constant of inactivation of CaV1.1a (blue, n = 6), CaV1.1a + γ1 (dark blue, n = 5), CaV1.1e (red, n = 6), and CaV1.1e + γ1 (dark red, n = 5) of a monoexponential fit on the decay phase of the inward calcium current during a 45-s depolarization to Vmax. (E) Example traces of 45-s depolarization to Vmax in CaV1.1a and CaV1.1e, normalized to the peak current. (F) The time constant of inactivation was accelerated by 33% (not significant) when CaV1.1a (P = 0.32) or CaV1.1e (P = 0.15) was coexpressed with γ1. Mean ± SEM; P values were calculated with Student’s t test.
The γ1 subunit increases membrane expression of both CaV1.1 isoforms
CaV1.1 is the only 1 out of the 10 voltage-gated calcium channels that expresses poorly in non-muscle cells, unless the adaptor protein STAC3 is coexpressed (Polster et al., 2015). Recently it was shown that the γ1 subunit also supports robust membrane expression of CaV1.1a in tsA201 cells; although in the absence of STAC3, these channels produce only very small calcium currents (Polster et al., 2016). To examine whether the γ1 subunit supports only the membrane targeting of the adult CaV1.1a isoform or also of the embryonic CaV1.1e, we established a dual-labeling approach, originally developed by the lab of Henry Colecraft (Fang and Colecraft, 2011; Yang et al., 2010), to identify and quantify membrane-inserted CaV1.1 channels. To this end, a 13 amino acid high-affinity BBS was introduced into the extracellular IIS5-IIS6 domain of GFP-CaV1.1a and GFP-CaV1.1e. Then, the channels expressed on the cell surface of HEK cells (expressing β3 and α2δ-1) were labeled by exposing unpermeabilized living cells to biotinylated BTX and subsequently to streptavidin-coated quantum dots (QD655; Fig. 5 A). Hence, the GFP fluorescence of a cell measures the total CaV1.1 expression, and the QD655 fluorescence quantifies the fraction of surface-expressed CaV1.1 channels.
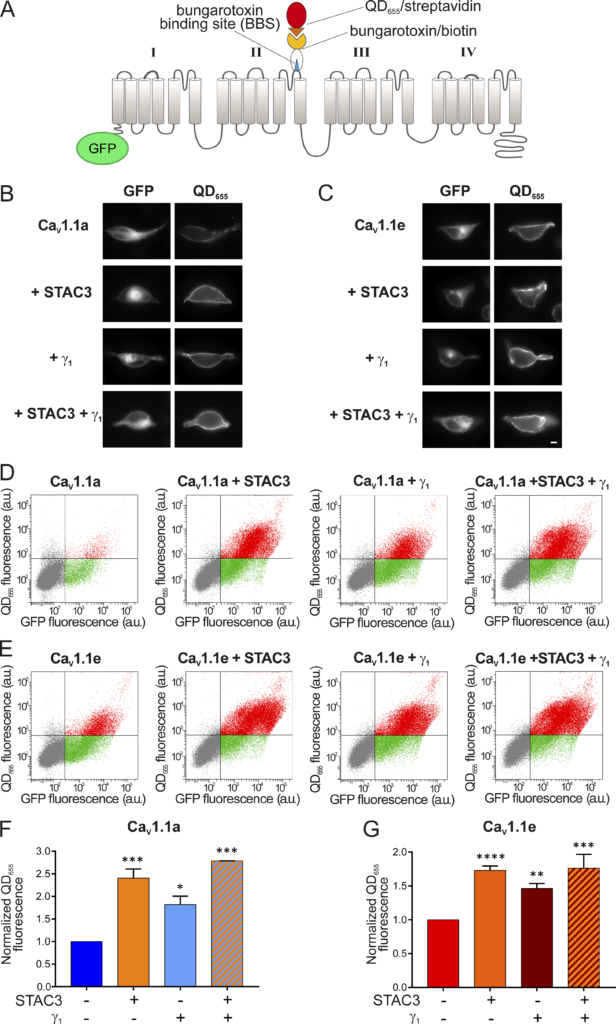
γ 1 I ncreases the surface density of both Ca V 1.1a and Ca V 1.1e isoforms. (A)
Scheme displaying the strategy to detect CaV1.1 channels expressed on the plasma membrane of HEK cells (stably expressing β3 and α2δ-1). The introduction of the 13 amino acid BBS in the extracellular domain of GFP-CaV1.1a or GFP-CaV1.1e allowed the selective labeling of channels in the membrane by sequentially incubating the unpermeabilized cells with biotinylated BTX and streptavidin-conjugated quantum dots (QD655). (B) From top to bottom, representative images of HEK cells expressing the adult GFP-CaV1.1a isoform alone, with STAC3, with γ1, and with both STAC3 and γ1. (C) The same for HEK cells expressing the embryonic GFP-CaV1.1e isoform. Scale bar, 2 µm. (D and E) Representative raw data from flow cytometry experiments showing the GFP and the QD655 signal for cells expressing GFP-CaV1.1a (D) or GFP-CaV1.1e (E) alone, with STAC3, with γ1, and with both STAC3 and γ1. The vertical and horizontal lines represent threshold values determined using untransfected cells, untreated cells, and cells exposed only to QD655. Single cells are depicted as dots, which have been colored in gray (untransfected), green (transfected, lacking surface expression), or red (transfected with appreciable surface expression). (F and G) Normalized mean QD655 fluorescence signals across separate flow cytometry experiments (n = 4). Data were normalized to the QD655 signals of cells expressing only GFP-CaV1.1. In F, the conditions with STAC3 (***, P = 0.0003), γ1 (*, P = 0.0143), and STAC3 + γ1 (***, P = 0.0002) are significantly different from the control GFP-CaV1.1a using one-way ANOVA and Tukey post-hoc mean comparison. In G, the conditions with STAC3 (****, P < 0.0001), γ1 (**, P = 0.0019), and STAC3 + γ1 (***, P = 0.0002) are significantly different from the control GFP-CaV1.1e using one-way ANOVA and Tukey post hoc mean comparison.
In cells expressing CaV1.1a alone, we detected minimal QD655 fluorescence in the plasma membrane. By contrast, coexpression of STAC3 or γ1, individually or together, resulted in robust CaV1.1a membrane targeting (Fig. 5 B). To quantify membrane-inserted CaV1.1 channels, we used flow cytometry analysis, which measures the fluorescence signals of a multitude of individual cells (Fig. 5 D). This analysis confirmed the lack of a robust QD655 fluorescence signal in cells expressing only GFP-CaV1.1a but the presence of a strong QD655 fluorescence in cells coexpressing GFP-CaV1.1a together with STAC3, γ1, or both. In four independent experiments, cells coexpressing STAC3 on average displayed a 140% increase of surface-expressed CaV1.1a, cells coexpressing γ1 displayed an 80% increase, and cells expressing both STAC3 and γ1 subunits displayed a 180% increase compared with cells expressing CaV1.1a alone (Fig. 5 F). These results corroborate the importance of STAC3 and γ1 for CaV1.1a plasma membrane expression (Niu et al., 2018; Polster et al., 2016; Polster et al., 2015).
We then analyzed the effect of the STAC3 and γ1 subunits on membrane expression of the embryonic CaV1.1e isoform. In contrast to the adult isoform, the embryonic CaV1.1e channel showed substantial membrane staining even when expressed alone (Fig. 5, C [top] and E [left]). Nevertheless, coexpression of STAC3 and γ1, individually or together, further increased the amount of QD655 fluorescence (Fig. 5, C and E). In four independent experiments, cells coexpressing STAC3 displayed a 70% increase of surface-expressed CaV1.1e, cells coexpressing γ1 displayed a 50% increase, and the ones expressing both STAC3 and γ1 subunits displayed an 80% increase compared with cells expressing CaV1.1e alone (Fig. 5 G).
Altogether, these results demonstrate that, although the γ1 subunit fails to modulate the current amplitude of the embryonic CaV1.1e isoform, it still modulates its steady-state inactivation and surface trafficking. Moreover, the reduction of current density induced by γ1 cannot be explained by reduced channel availability at the cell surface.
CaV1.1–γ1 ion-pair partners predicted by structure modeling are not essential for CaV1.1a-specific current reduction by γ1
Because the recent cryo-EM structure of CaV1.1 revealed that the γ1 subunit interacts with IVS3-S4 (Wu et al. 2016; Wu et al. 2015), and because we found that γ1 fails to inhibit the current amplitude of the embryonic CaV1.1e isoform (Fig. 2 E), which lacks 19 amino acids in the IVS3-S4 linker, we hypothesized that γ1 and the IVS3-S4 linker of CaV1.1a may establish an interaction responsible for the current inhibition in CaV1.1a. To identify putative interaction partners between the IVS3-S4 linker and γ1, we generated a structural model of the CaV1.1 channel based on the published cryo-EM structure (Wu et al., 2016; Fig. 6).
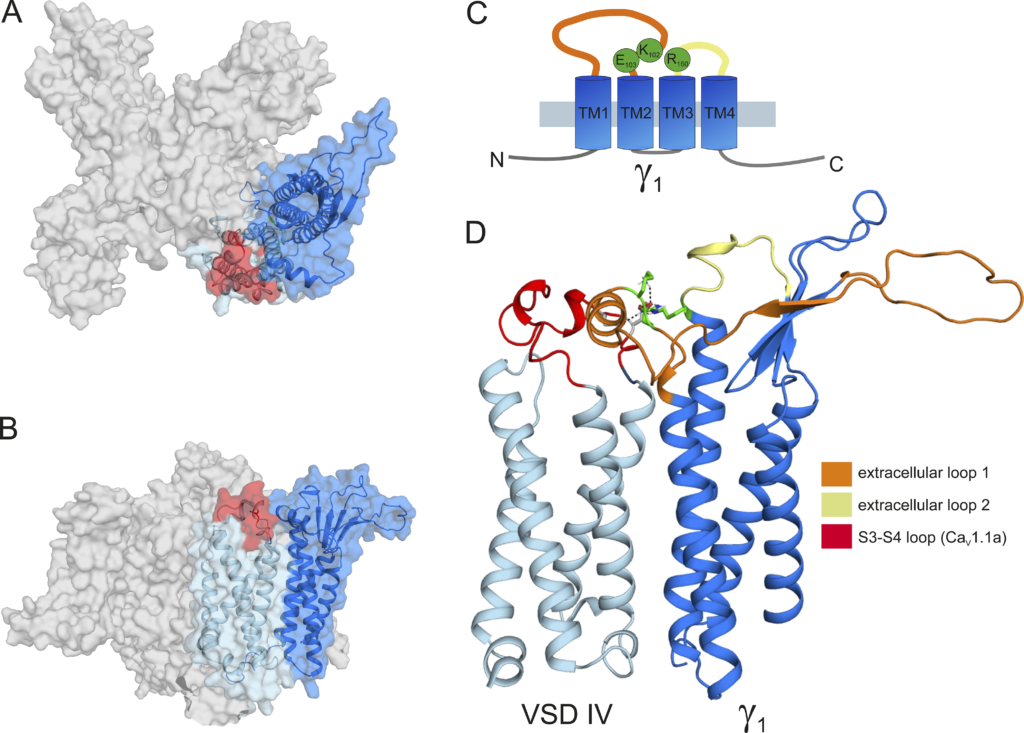
Structure modeling of Ca V 1.1a in complex with the γ 1 subunit. (A)
Top view of the structure model of the human CaV1.1 α1 subunit in complex with the γ1 subunit refined with molecular dynamics simulation in a membrane environment based on the 3.6-Å structure of rabbit CaV1.1 (Wu et al., 2016). VSD IV (light blue) is interacting with the γ1 subunit (marine). The alternatively spliced exon 29 (red) is inserted in the IVS3-S4 linker of CaV1.1a. (B) Side view of the structure model of CaV1.1 with the γ1 subunit. (C) Cartoon showing the domain organization of γ1, with the mutated residues R160, K102, and E103 in green. (D) Close-up of the interaction site of the VSD IVS3-S4 loop with the γ1 subunit, highlighting the extracellular loops of the γ1 subunit. The extracellular loop 1 of γ1 is in the same orientation as presented in Fig. 7, A, E, and M, and residue R160 is highlighted in green.
We used Rosetta computational modeling software (Bender et al., 2016; Rohl et al., 2004) to model the structure of the IVS3-S4 linker of CaV1.1a. The resulting structure predicts a putative interaction of residues D1223 and D1225 of the IVS3-S4 linker of CaV1.1a with residue R160 in the second extracellular loop of the γ1 subunit (Figs. 7 A and 6 B). To test whether the observed inhibition of the CaV1.1a current amplitude by γ1 is dependent on this ionic interaction, we performed site-directed mutagenesis to substitute the involved residues with alanines, which deletes all interactions made by side-chain atoms beyond the β carbon (Wells, 1991). However, mutation of residue R160 of the γ1subunit to an alanine did not diminish its ability to inhibit the current amplitude of CaV1.1a (Fig. 7, A–D; and Table 3). Also, simultaneously mutating both D1223 and D1225 of CaV1.1a did not alter the ability of γ1 to reduce the current amplitude of CaV1.1a (Fig. 7, E–H; and Table 3). Together, these results indicate that this putative interaction between the IVS3-S4 linker of CaV1.1a and the γ1 subunit is dispensable for current amplitude inhibition by γ1.
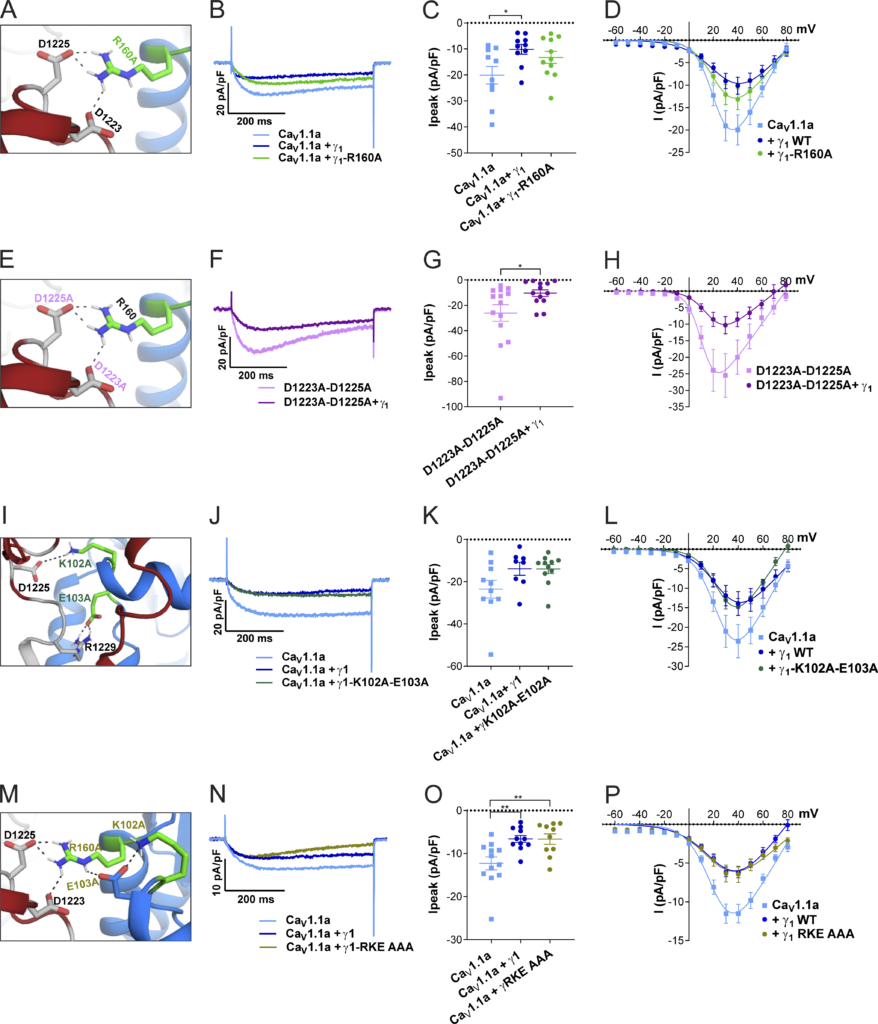
The putative interactions between the IVS3-S4 loop and γ 1 identified by structure modeling are dispensable for Ca V 1.1a current reduction. (A–H)
Structure modeling of CaV1.1a and γ1indicates interactions of R160 (γ1) with D1223 and D1225 (CaV1.1a). Neutralizing the putative CaV1.1a interaction partner (R160A; A) or the γ1 interaction partners (D1223A and D1225A; E), did not impair current reduction by γ1 (B–D and F–H). (I–L) Structure modeling of CaV1.1a and γ1 indicates further interactions of K102 and E103 (γ1) with D1225 and R1229 (CaV1.1a). Neutralizing both of these putative CaV1.1a interaction partners to alanine (K102A/E103A; I) did not abolish the ability of γ1 to reduce CaV1.1a current (J and K). (M–P) Concomitant mutation of all three γ1 residues involved in these putative interactions did not abolish the current reduction by γ1 (N–P). Exemplary current traces at Vmax (B, F, J, and N); scatter plots of Ipeak (C, G, K, and O); and current–voltage relationship (D, H, L, and P). Mean ± SEM; P values calculated with ANOVA and Tukey’s post-hoc test. *, P < 0.05; **, P < 0.01.
Previously, it has been suggested that the N-terminal half of the γ1 subunit, including the first two transmembrane domains, mediates its interaction with the calcium channel and is responsible for suppressing the current amplitude of CaV1.1 (Arikkath et al., 2003). Because the analyzed R160A mutation is located outside of this region in the C-terminal half of the γ1 subunit protein, we further modeled the structure of the extensive extracellular loop located in the first half of the γ1 subunit and searched it for possible interaction sites with the IVS3-S4 linker of CaV1.1a. We identified putative ionic interactions of residues D1225 and R1229 in the IVS3-S4 linker of CaV1.1a with K102 and E103 positioned in the first extracellular domain of the γ1 subunit (Fig. 7 I, Table 3, and Fig. 6). However, mutation of K102 and E103 to alanines did not alter the ability of γ1 to inhibit the calcium channel current amplitude (Fig. 7, J–L; and Table 3). Finally, to exclude the possibility that the interaction between the IVS3-S4 linker of CaV1.1a with either one of the two extracellular loops of γ1 was sufficient to suppress the calcium channel current amplitude, we combined the R160A and K102A/E103A mutations (Fig. 7 M). However, this triple-mutant γ1 was also capable of inhibiting the current amplitude of CaV1.1a to levels similar to the wild type γ1 (Fig. 7, N–P; and Table 3). Together, these mutagenesis experiments suggest that the current-inhibiting effect of γ1 is not mediated by the direct ionic interactions between γ1 and the IVS3-S4 loop of CaV1.1a, at least not those predicted by our structure modeling.
Discussion
Whereas the role of the auxiliary α2δ and β subunits in subcellular targeting and gating modulation have been extensively studied for high voltage activated calcium channels in heterologous cells, this has not been the case for the γ1 subunit. γ1 is a specific subunit of the skeletal muscle CaV1.1 isoform and, until recently, CaV1.1 had resisted efficient functional expression in heterologous expression systems. Only since the discovery of STAC3 as an essential component of the CaV1.1 channel complex, permitting the reliable heterologous expression of CaV1.1, have such analyses been possible (Horstick et al., 2013; Nelson et al., 2013; Polster et al., 2015). Here, we developed and validated two HEK cell lines stably expressing STAC3 (plus α2δ-1 and β3), which proved to be a convenient and efficient heterologous expression system for CaV1.1. By coexpression of CaV1.1 and γ1 in these cells, we found three effects of the γ1 subunit: facilitated membrane expression of CaV1.1, a reduction of the current density, and a shift of steady-state inactivation to hyperpolarizing potentials. The effects of the γ1 subunit on the two splice variants of CaV1.1 expressed in our new STAC3-HEK cell lines revealed a novel, isoform-dependent mechanism of channel modulation by this subunit. Although γ1 equally supports membrane expression of CaV1.1a and CaV1.1e, it functions only as a negative regulator of the adult CaV1.1a splice variant. This differential regulation of current density is mediated by the inclusion of the alternatively spliced exon 29 in the extracellular loop connecting helices S3 and S4 in repeat IV, but it does not require the direct ionic interactions between this loop and the γ1 subunit. Another novel finding is that in both the adult and embryonic CaV1.1 splice variants, γ1reduces steady-state inward current at more negative voltages by shifting the voltage dependence of steady-state inactivation, but not of activation, to more negative voltages and by promoting the time course of current inactivation.
The γ1 subunit supports membrane expression of CaV1.1
The substantially increased surface expression induced by coexpression of γ1observed with extracellular BTX labeling and flow cytometry did not translate into increased current densities. This is consistent with the observation that in γ1-null mouse muscle, in which STAC3 is endogenously expressed, the expression levels of CaV1.1 are similar to those of wild type mice (Arikkath et al., 2003). In our experiments, this is explained by the observation that the effects of γ1 and STAC3 on membrane expression are not additive, and therefore γ1 does not significantly increase CaV1.1 beyond the level already achieved by STAC3. Apparently, an independent component must be limiting for membrane targeting. The effect of γ1on membrane targeting in heterologous cells is consistent with a previous immunocytochemistry and charge movement analysis showing that in the absence of STAC3, the γ1 subunit supports robust membrane expression of CaV1.1 in tsA201 cells, while sustaining only very small currents (Polster et al., 2016). In contrast, an earlier Western blot analysis of tsA201 cells lysates reported that coexpression of γ1reduces the levels of CaV1.1 protein expression (Sandoval et al., 2007). In sum, our results corroborate the findings that the γ1 subunit supports membrane expression of CaV1.1 in heterologous cell systems in a splice variant–independent manner, possibly by masking retention motifs on the C-terminus (Niu et al., 2018), but without adding to the calcium influx.
The γ1 subunit promotes steady-state inactivation in CaV1.1a and CaV1.1e
Functionally, the two negative actions of γ1 on CaV1.1 currents dominate. The observed decrease in current amplitude and left-shift of steady-state inactivation are in general agreement with previous studies in muscle cells (Ahern et al., 2001; Freise et al., 2000) as well as in tsA201 cells expressing CaV1.1a (Polster et al., 2016). Limiting calcium influx through CaV1.1 during muscle activity is tolerable because of the principal role of CaV1.1 as a voltage sensor in skeletal muscle EC coupling (Schneider and Chandler, 1973; Rios and Brum, 1987). At the same time, it is important to limit interference of calcium influx with other calcium signaling events, such as those regulating fiber type specification, and to avoid adverse effects of calcium overload on mitochondrial integrity (Sultana et al., 2016). Previously, we pointed out how intrinsic mechanisms in the CaV1.1 α1S subunit and the actions of auxiliary subunits cooperate in limiting the calcium currents in skeletal muscle (Tuluc et al., 2009; Flucher et al., 2005). Whereas the α2δ-1 subunit slows down the activation, the γ1 subunit promotes voltage-dependent inactivation at more negative voltages. This effect was equally observed in the adult and, as shown here for the first time, the embryonic splice variant. Together with the observed increase in membrane targeting, this is the first experimental evidence demonstrating that the γ1subunit functionally interacts with the embryonic splice variant CaV1.1e. Therefore, this modulatory effect is independent of the length of the extracellular loop connecting helices IVS3 and IVS4.
The γ1 subunit reduces the current amplitude specifically in CaV1.1a
The most interesting finding of this study is the differential down-regulation of calcium currents in CaV1.1a versus CaV1.1e. The small current size is one of the hallmarks of skeletal muscle calcium currents. Our results demonstrate that the γ1subunit is a major determinant of this reduced current density. Whereas in skeletal muscle the adult and embryonic CaV1.1 splice variants differ substantially in voltage dependence of current activation and in current size, the currents recorded in the HEK cells (stably expressing α2δ-1, β3, and STAC3) reproduced the difference in V1/2of activation, but not in current density. Apparently, this difference is due to the lack of one or more muscle-specific factors in the heterologous expression system. As coexpression of γ1 restored the reduced current density in CaV1.1a compared with CaV1.1e, the γ1 subunit is such a factor. Quantitatively, the difference in current density between the two splice variants was still smaller than that observed when the same constructs were expressed in dysgenic myotubes (Tuluc et al., 2016; Tuluc et al., 2009). Therefore, it is likely that other modulatory mechanisms present in the native environment of the channel in the skeletal muscle triads contribute to the full expression of this splice variant–specific difference. The γ1 subunit is one of two proteins shown to differentially modulate the current properties of the two CaV1.1 splice variants, along with RYR1 (Benedetti et al., 2015), demonstrating the importance of the native cellular environment for the accurate expression of physiological current properties. Notably, γ1 does not reduce the current density of CaV1.1a by decreasing its plasma membrane expression. As previously shown, CaV1.1e has a higher open probability than CaV1.1a in skeletal myotubes (Tuluc et al., 2009). Therefore, the most likely explanation is that γ1 decreases the channel’s maximal open probability in a splice variant–specific manner.
The sole difference in the primary structure between the embryonic and adult splice variants is the inclusion of 19 amino acids coded in exon 29 in the IVS3-S4 loop of CaV1.1a. Apparently, this difference determines the action of the γ1 subunit on current size. There are two possible mechanisms how inclusion of exon 29 can enable this functional interaction with γ1: direct interactions between the IVS3-S4 loop and γ1 or the stabilization of a conformation of the channel complex by inclusion of exon 29 that renders CaV1.1a susceptible to this particular γ1modulation. As the first possibility is amenable to experimental testing, we examined it by identifying and mutating putative interaction sites on both channel subunits. However, none of these ion pairs seemed to be essential for the current-reducing action of γ1. This result is in agreement with previous findings showing that CaV1.1 current reduction is mediated by the first two transmembrane domains of γ1, and that the extracellular loop is dispensable for this interaction (Arikkath et al., 2003). Therefore, it is unlikely that this effect is mediated by the direct interaction of the γ1 subunit with the IVS3-S4 loop, although our experiments do not entirely rule out this possibility.
Given that the CaV1.1 structure identified the II and III transmembrane domains of γ1as the ones involved in the interaction with the IVS3-S4 of CaV1.1 (Wu et al., 2016), and that the first two transmembrane domains of γ1 were sufficient for reconstituting the current reduction (Arikkath et al., 2003), we can deduce that an interaction between the second transmembrane domain of γ1 and the IVS4 of CaV1.1 is the most likely scenario for mediating the current-reducing effect. We therefore conclude that insertion of exon 29 into the IVS3-S4 loop alters the conformation of the channel in a way that enables it to respond to the inclusion of γ1 with a reduced current density (Fig. 8 A). Notably, the left-shifted activation in CaV1.1e compared with CaV1.1a is observed with or without γ1, and the left-shifted inactivation is observed with or without exon 29, whereas the decreased current amplitude requires their cooperation. Evidently, the interdependence of the analyzed gating properties on the IVS3-S4 loop and the γ1 subunit is highly specific. Each of the partners independently exerts its specific action on the voltage dependence of activation and inactivation (Fig. 8 B).
Uncoupling of the effects of the γ1 subunit on current size and inactivation
The finding that the current amplitude of the embryonic variant CaV1.1e is not modulated by the γ1 subunit, unlike the adult isoform, is surprising. In fact, in a previous study in γ1 knockout mice, it was reported that the difference in current density between wild type and knockout mice is age dependent, as it was detected only in mice <4 wk of age, but not in older animals (Held et al., 2002). However, this observation cannot be explained by the differential current regulation of CaV1.1a and CaV1.1e reported here. There is no evidence that primary cultures derived from muscles at different times after birth express different ratios of CaV1.1e and CaV1.1a. Moreover, if there were such differences, muscles of mice at ≥4 wk would be expected to express predominantly the adult isoform and thus be more susceptible to modulation by γ1 than younger muscles, not the opposite (Tang et al., 2012; Sultana et al., 2016; Tuluc et al., 2009)
More importantly, unlike the age-dependent reduction in current amplitude, in muscles of γ1 knockout mice, the shift in the steady-state inactivation was found to be age independent (Held et al., 2002), suggesting that these two functional effects of the γ1 subunit are not coupled with each other. Here, we observed a similar lack of coupling of the two γ1 effects in CaV1.1e, which is subject to the shift in steady-state inactivation but not to the reduction in current density in cells coexpressing γ1. Together, these data strongly suggest that the two γ1 functional effects are independent of each other and possibly mediated by different domains.
The role of the γ1 subunit in orthograde and retrograde coupling of CaV1.1 and RYR1
The γ1 subunit was previously demonstrated to be dispensable for EC coupling, i.e., the orthograde coupling between CaV1.1 and the RYR1. In fact, in γ1-null myotubes, neither the amplitude nor the voltage dependence of the calcium transients was affected (Ahern et al., 2001; Freise et al., 2000). Likewise, calcium release was unaffected in γ1-null myotubes, and twitch and tetanic force development of adult γ1-null mice was very similar in both fast and slow muscles (Ursu et al., 2001). However, long-lasting potassium-induced contractures were significantly larger, and the shift of the steady-state inactivation in CaV1.1 currents was shown to translate into a similar shift in the inactivation curve of calcium release of adult skeletal muscle fibers (Ursu et al., 2004). Our finding that γ1 equally shifts the voltage dependence of inactivation of CaV1.1a and CaV1.1e indicates that the corresponding shift in the inactivation curve of calcium release also may be present.
In skeletal muscle, not only does CaV1.1 activate RYR1, but CaV1.1a calcium currents are also augmented by an interaction of its cytoplasmic II–III loop with the RYR1 (Grabner et al., 1999), a phenomenon termed retrograde coupling. Previously we demonstrated that this function is specific for the adult CaV1.1a splice variant (Benedetti et al., 2015). The currents of CaV1.1e are not reduced when the connection with RYR1 is severed. The dependence of the current augmentation by retrograde coupling on inclusion of exon 29 in the IVS3-S4 loop of CaV1.1 mirrors the importance of exon 29 for the current reduction by γ1. Based on the results of the earlier study, we had proposed a mechanistic model according to which retrograde coupling partially relieves the inhibition of CaV1.1 currents by an unknown, exon 29–dependent factor. Our current study suggests that the γ1 subunit may be this inhibitory factor. According to this hypothetical model, in the simultaneous presence of exon 29 and the γ1 subunit, the currents of CaV1.1a are reduced, and this effect is partially counteracted by the interaction with RYR1. If either exon 29 or the γ1 subunit is missing, this inhibition is absent and there is nothing to be relieved by retrograde coupling (Fig. 8 C).
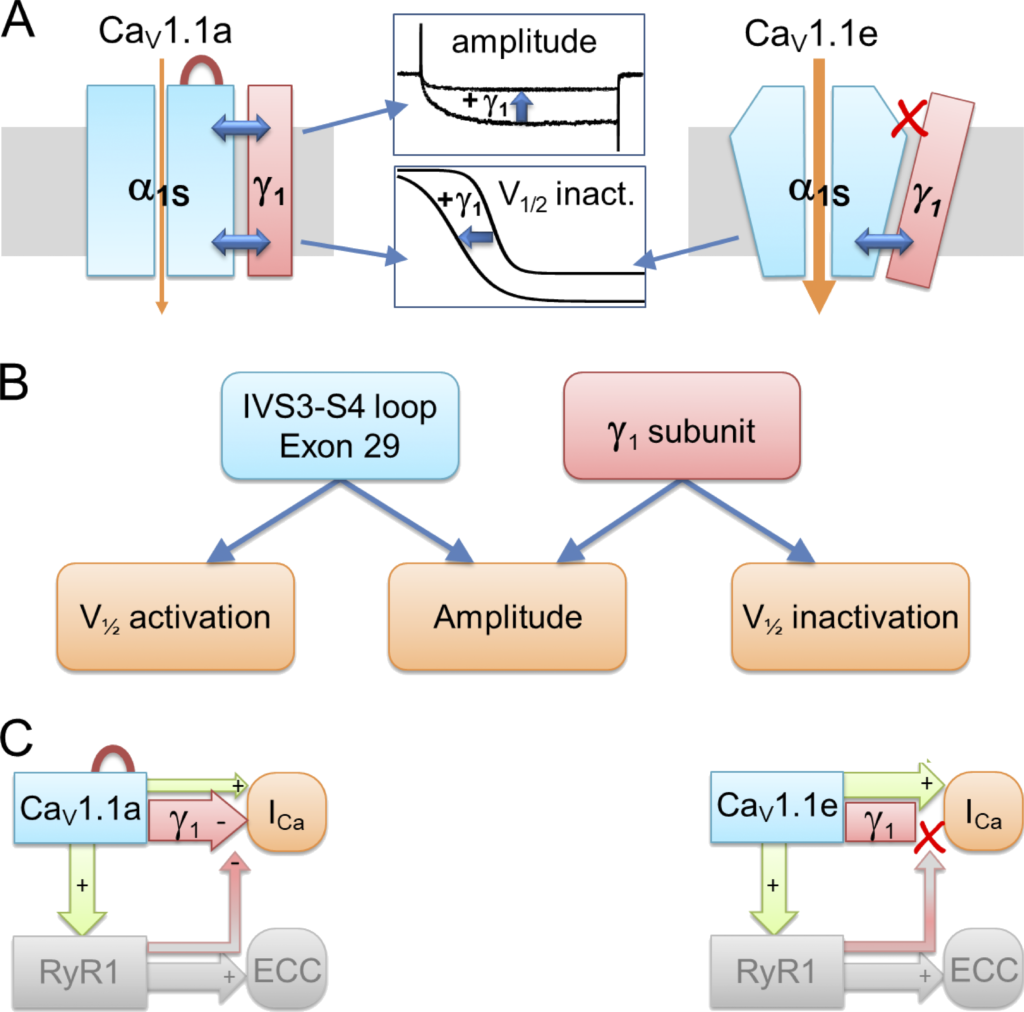
Model of differential γ 1 modulation on Ca V 1.1a and Ca V 1.1e currents and its consequences for retrograde coupling. (A)
In both CaV1.1 splice variants, the γ1 subunit limits calcium currents by shifting the voltage dependence of inactivation to more hyperpolarizing potentials and rendering inactivation more complete. Inclusion of exon 29 in the extracellular IVS3-S4 loop stabilizes a conformation of the CaV1.1a channel complex, which enables the γ1 subunit to reduce the current amplitude. (B) The IVS3-S4 loop including exon 29 and the γ1 subunit require each other for reducing the current amplitude. In contrast, this cooperation is not required to shift the voltage dependence of activation and inactivation, which occurs in a splice variant–dependent manner. (C) In skeletal muscle cells, the negative regulation of calcium currents by the γ1 subunit is a prerequisite of retrograde current amplification by the RYR1 in CaV1.1a (red arrow from RYR1 to γ1; Grabner et al., 1999; Nakai et al., 1996). Without exon 29 in embryonic CaV1.1e, no γ1-dependent reduction of current amplitude and no RYR1-dependent relief of this inhibition occurs (Benedetti et al., 2015). The red loop in CaV1.1a indicates inclusion of exon 29.
Conclusions
This analysis of the actions of the γ1 subunit on the two splice variants of CaV1.1 in heterologous cells revealed multiple functions of γ1 in membrane targeting and functional modulation of the skeletal muscle calcium channel. Interestingly, some of the γ1 effects are general for both splice variants, while another is specific for the adult CaV1.1a. Inclusion of exon 29 in CaV1.1a appears to allosterically render the channel susceptible to the reduction of its currents by γ1, as well as to the simultaneous relief of this block by RYR1. Newly generated mammalian cell systems proved highly valuable for this type of coexpression study of CaV1.1, but at the same time highlight the multitude of factors involved in shaping the physiological current properties in the native environment of skeletal muscle.